
Am Fam Physician. 2005;71(5):903-910
Group B streptococcus (GBS) is a leading cause of morbidity and mortality among newborns. Universal screening for GBS among women at 35 to 37 weeks of gestation is more effective than administration of intrapartum antibiotics based on risk factors. Lower vaginal and rectal cultures for GBS are collected at 35 to 37 weeks of gestation, and routine clindamycin and erythromycin susceptibility testing is performed in women allergic to penicillin. Women with GBS bacteriuria in the current pregnancy and those who previously delivered a GBS-septic newborn are not screened but automatically receive intrapartum antibiotics. Intrapartum chemoprophylaxis is selected based on maternal allergy history and susceptibility of GBS isolates. Intravenous penicillin G is the preferred antibiotic, with ampicillin as an alternative. Penicillin G should be administered at least four hours before delivery for maximum effectiveness. Cefazolin is recommended in women allergic to penicillin who are at low risk of anaphylaxis. Clindamycin and erythromycin are options for women at high risk for anaphylaxis, and vancomycin should be used in women allergic to penicillin and whose cultures indicate resistance to clindamycin and erythromycin or when susceptibility is unknown. Asymptomatic neonates born to GBS-colonized mothers should be observed for at least 24 hours for signs of sepsis. Newborns who appear septic should have diagnostic work-up including blood culture followed by initiation of ampicillin and gentamicin. Studies indicate that intrapartum prophylaxis of GBS carriers and selective administration of antibiotics to newborns reduce neonatal GBS sepsis by as much as 80 to 95 percent.
Group B streptococcus (GBS), or Streptococcus agalactiae, is one of the leading causes of morbidity and mortality among newborns, resulting in sepsis, pneumonia, and meningitis. During the past decade, major initiatives have been proposed to prevent early-onset infection, which is defined as disease occurring in newborns younger than seven days.1 The goal of preventive strategies is to reduce or eliminate transmission of GBS to the neonate by giving antibiotics to GBS-colonized women during delivery and selectively administering antibiotics to newborns after delivery. Despite strict implementation, no strategy will prevent all cases of neonatal GBS sepsis.2 However, critical reviews of the literature demonstrate that intrapartum maternal prophylaxis alone3 or combined with postpartum neonatal prophylaxis4 reduces early-onset attack rates by 80 percent and 95 percent, respectively.
| Key clinical recommendation | Label | References |
|---|---|---|
| Universal screening at 35 to 37 weeks of gestation and intrapartum treatment of colonized women are the most effective approaches in reducing GBS sepsis of the newborn. | A | 3,21 |
| Rectovaginal cultures for GBS should be performed one to five weeks before delivery. | C | 26 |
| Intrapartum antibiotic prophylaxis is not indicated if an elective cesarean delivery is planned and there is no labor or rupture of membranes. | C | 13 |
| Routine susceptibility testing of GBS isolates should be performed. | C | 28 |
| The practice of combining protocols such as administering intrapartum prophylaxis to GBS-negative women with ruptured membranes more than 18 hours before delivery is not recommended. | C | 13,23 |
| The first choice for intrapartum antibiotic prophylaxis is intravenous penicillin G. | C | 13 |
| GBS-positive women at high risk of anaphylaxis should receive clindamycin instead of penicillin G or cefazolin. | C | 13 |
| Neonates who appear to be septic and those born to mothers with chorioamnionitis should receive a diagnostic work-up including blood culture, CBC with differential, and possible lumbar puncture before antibiotics are initiated. | C | 13 |
Epidemiology and Risk Factors
In the mid 1980s, it was demonstrated that GBS was carried in the vaginal and anorectal flora of up to 30 percent of women.5 Maternal colonization can be intermittent, transient, or persistent.6 Fortunately, the attack rate in newborns is low. The incidence of early-onset neonatal disease is one to two cases per 1,000 live births, with a mortality rate of up to 20 percent in affected neonates.7,8 Although attempts have been made to identify risk factors that influence the prevalence of GBS, such as ethnicity, smoking, maternal age, and number of partners,9 the colonization rates are inconsistent enough that targeting only high-risk women for selective screening is not an effective strategy. Compared with infants born to lightly colonized women, those born to heavily colonized women have 2.5 times the risk of infection.10 Neonates born to mothers who have GBS bacteriuria at any time during pregnancy are known to be more frequently and more heavily colonized with GBS and are more likely to develop sepsis.11
| Chorioamnionitis |
| GBS bacteriuria in current pregnancy |
| Maternal rectovaginal colonization |
| Maternal temperature of 38.0°C (100.4°F) or greater |
| Preterm labor or preterm rupture of membranes at less than 37 weeks of gestation |
| Previous delivery of infant with early-onset GBS sepsis |
| Prolonged interval (18 hours or more) between rupture of membranes and delivery |
Evolution of Guideline Recommendations
In the 1980s, researchers found that effective treatment of GBS-colonized women resulted in reduced rates of neonatal colonization and sepsis.15 In 1996, the Centers for Disease Control and Prevention (CDC) stated that one of the following two preventive strategies could be used: (1) universal prenatal screening of all women at 35 to 37 weeks of gestation followed by intrapartum chemoprophylaxis of all GBS carriers, or (2) treatment of women in labor who develop risk factors and whose GBS status is unknown.16 The American College of Obstetricians and Gynecologists (ACOG) supported the CDC recommendations.17
At the time, both strategies, referred to as culture-based or risk-based, were considered equally effective in preventing neonatal GBS sepsis. Hospitals that adopted the recommendations had fewer GBS-infected neonates.18 Before and after implementation of a combined maternal and neonatal protocol at one hospital, the number of cases of early-onset disease dropped from 31 to six live births per 13,500 women, or from 2.2 to 0.4 per 1,000 live births, an 80 percent reduction.19,20 Additionally, compared with white neonates, the excess incidence of GBS sepsis among black newborns decreased by 75 percent over a five-year period. Other estimates suggest that during the same period, implementation of these strategies prevented 3,900 neonatal infections and 200 neonatal deaths.
A systematic review of the literature showed that although the risk-factor approach is the least expensive option, universal screening at 35 to 37 weeks of gestation and treating all colonized women during labor are the most effective options.21 Neonatal GBS sepsis is reduced by 78 percent when using the screening protocol compared with 41 percent using the risk-based method.21
Another comparison of the two approaches revealed that the screening approach was more effective by one half than the risk-based approach in preventing neonatal GBS disease.22 The efficacy of intrapartum chemoprophylaxis in preventing neonatal GBS in infants of colonized women without clinical risk factors was 89 percent. Therefore, in 2002, the CDC13 and ACOG23 issued guidelines recommending the universal screening protocol. The CDC revision included guidelines for selective neonatal antibiotic prophylaxis.
GBS Cultures
Maternal culturing between 35 and 37 weeks of gestation is recommended. Both anorectal and vaginal cultures are collected because the rectum is the natural reservoir for GBS. The yield of vaginal cultures for GBS is only 60 percent of the yield of vaginal and rectal cultures combined.24 Use of a speculum is not required. Sampling the cervix or vaginal fornices results in a significantly lower yield and is not recommended.25 For the single-swab method, the lower one third of the vagina is swabbed circumferentially with a cotton swab that is then inserted through the anal sphincter 2 cm into the rectum and rotated 360 degrees. If preferred, a two-swab technique can be used. The sample is then placed directly into transport media that can be kept up to four days at room temperature or refrigerated.
A systematic review26 demonstrated that the sensitivity and specificity of rectovaginal cultures at 36 weeks of gestation were 91 and 89 percent, respectively. The sensitivity and specificity of cultures obtained one to five weeks before delivery were 87 and 97 percent, respectively. In contrast, the sensitivity and specificity of cultures taken six or more weeks before delivery for predicting colonization status at delivery were 43 and 85 percent.27 Therefore, cultures obtained more than five weeks before delivery may not accurately predict colonization at delivery.
Resistance Patterns
With as many as 25 percent of laboring women receiving chemoprophylaxis for GBS, the possibility of emerging resistance to the most commonly used antibiotics is a concern, especially if penicillin allergy is present. Therefore, it is recommended that routine susceptibility testing of GBS isolates be performed.28 Several patterns of isolate resistance have emerged. A 1998 study indicated a 15 percent resistance to clindamycin (Cleocin); a 16 percent resistance to erythromycin; and no resistance to penicillin G, vancomycin (Vancocin), or cefazolin (Ancef).29 In 2001, an 8 percent resistance to clindamycin and a 19 percent resistance to erythromycin were demonstrated.30 All isolates were susceptible to vancomycin while resistance to penicillin and cefazolin was less than 2 percent.
Indications for Intrapartum Antibiotic Prophylaxis
Indications for intrapartum chemoprophylaxis include maternal antenatal GBS bacteriuria in any concentration in the current pregnancy, previous delivery of an infant with GBS disease, and positive maternal GBS cultures at 35 to 37 weeks of gestation (Table 2).13 Treatment following usual seven-day protocols for urinary tract infection should be initiated at the time of prenatal diagnosis of GBS bacteriuria, making subsequent screening at 35 to 37 weeks of gestation unnecessary.
| GBS bacteriuria during current pregnancy (no need for rectovaginal culture at 35 to 37 weeks of gestation) | |
| GBS status unknown within six weeks of delivery and any of the following: | |
| Less than 37 weeks of gestation and cesarean delivery not planned | |
| Rupture of membranes 18 hours or more before delivery | |
| Maternal temperature of 38.0°C (100.4°F) or greater and no evidence of chorioamnionitis | |
| Positive maternal GBS culture at 35 to 37 weeks of gestation during current pregnancy | |
| Previous newborn with GBS-invasive disease | |
Antibiotic prophylaxis is indicated if the GBS status of the mother is unknown within six weeks of delivery and if risk factors develop (Table 2).13 Rectovaginal cultures should be obtained in women with preterm labor or with preterm rupture of membranes before antibiotics are begun, unless they are known to be GBS positive (Table 3).13,23 Intrapartum prophylaxis is not indicated if an elective cesarean section is planned and there is no labor or rupture of membranes, regardless of GBS status documented at 35 to 37 weeks of gestation (Table 4).13 GBS colonization in a previous pregnancy is not an indication for treatment in the current pregnancy.13
| GBS status | Recommended course of action |
|---|---|
| GBS negative (within six weeks) | No GBS prophylaxis |
| GBS positive | Start GBS prophylaxis* continuing for at least 48 hours during tocolysis and during labor. |
| GBS unknown (or no GBS culture in past six weeks) | Obtain rectovaginal GBS culture. Start GBS prophylaxis* and, if no culture growth in 48 hours, stop antibiotic. |
| Negative rectovaginal GBS culture at 35 to 37 weeks of gestation (regardless of intrapartum risk factors) |
| Planned cesarean delivery performed in the absence of labor or membrane rupture (regardless of maternal GBS status) |
| Previous pregnancy with positive GBS screening culture with a negative culture during the current pregnancy |
Chemoprophylaxis
MATERNAL REGIMENS
The choice of intrapartum prophylactic antibiotics for GBS-positive women depends on maternal allergy history and antibiotic susceptibility (Table 5,Figure 1).13 The first choice is intravenous penicillin G, 5 million units initially followed by 2.5 million units every four hours until delivery.13 Penicillin G is preferred because of its narrow spectrum profile, although ampicillin is an acceptable alternative if penicillin G is unavailable. Ideally, antibiotics should be administered at least four hours before delivery to achieve adequate placental and amniotic fluid concentration. When the time between the start of antibiotics and delivery is less than one hour, the rate of GBS transmission is 46 percent compared with 1.2 percent if the interval is more than four hours.31
| Regimens | Antimicrobial | |
|---|---|---|
| Recommended | Penicillin G—initial dose: 5 million units IV | |
| Subsequent dosing: 2.5 million units IV every four hours until delivery | ||
| Alternative (if penicillin G not available) | Ampicillin—initial dose: 2 g IV | |
| Subsequent dosing: 1 g IV every four hours until delivery | ||
| Penicillin allergy | Clindamycin (Cleocin)—900 mg IV every eight hours until delivery | |
| and | or | |
| High risk* for anaphylaxis | Erythromycin—500 mg IV every six hours until delivery | |
| and | ||
| GBS susceptible to clindamycin and erythromycin | ||
| Penicillin allergy | Vancomycin (Vancocin)—1 g IV every 12 hours until delivery | |
| and | ||
| High risk* for anaphylaxis | ||
| and | ||
| GBS resistance to clindamycin or susceptibility unknown | ||
| Penicillin allergy | Cefazolin (Ancef)—initial dose: 2 g IV | |
| and | Subsequent dosing: 1 g IV every eight hours until delivery | |
| Not at high risk* for anaphylaxis† | ||
Penicillin allergies are common, but are likely over-reported or misinterpreted by the patient.32 Before determining whether an alternative drug is necessary, the nature of the reported reaction to penicillin should be confirmed.13 For women with a history of penicillin allergy other than immediate hypersensitivity reactions, cefazolin is the preferred antibiotic because its transplacental transfer is similar to that of ampicillin.13,33
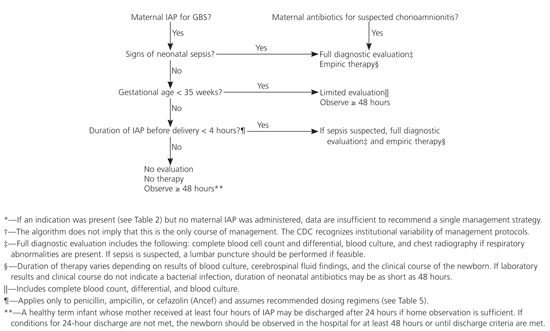
GBS-positive women at high risk of anaphylaxis should not receive penicillin G or cefazolin because of the risk of cross-reactivity, but rather they should receive intravenous clindamycin in a dosage of 900 mg every eight hours until delivery.13 Although listed as an option by the CDC, erythromycin is less preferred because of higher resistance rates and poor placental transfer.34 Vancomycin is reserved for women allergic to penicillin who are at high risk of anaphylaxis and for whom GBS antibiotic susceptibility testing has revealed resistance to clindamycin and erythromycin.
SPECIAL CONSIDERATIONS
There will still be the rare occasion when a laboring patient has unknown GBS status (because of missed appointments, lost or mislabeled specimen, laboratory error, etc.). Because the risk-based approach has been shown to be cost-effective and reduce neonatal GBS infection, it is preferred in this circumstance.13
While intrapartum chemoprophylaxis focuses on GBS, non-GBS infections in neonates also can cause significant morbidity and/or mortality. However, there is no apparent increased risk of non-GBS infections in term infants with the current policy of intrapartum chemoprophylaxis.35
NEWBORN REGIMENS
Empiric, universal antibiotic administration to neonates, regardless of maternal colonization status, is not recommended, because it is associated with a 40 percent increase in overall neonatal mortality despite reducing the GBS attack rate by 68 percent.4 Selective neonatal prophylaxis is recommended13 when maternal GBS carriage has been documented but less than four hours of antibiotic prophylaxis have been given before delivery, chorioamnionitis is suspected, or signs of neonatal sepsis are present.4
Maternal prophylaxis reduces, but does not eliminate, the risk of neonatal sepsis. Because the signs of sepsis in newborns can be nonspecific and insensitive, a conservative approach to management has been advocated. Closely observing newborns during transition is necessary to recognize signs of sepsis. Ninety-five percent of neonates with GBS-positive infections and signs of sepsis exhibit them in the first 24 hours of life.36 When neonatal outpatient follow-up is sufficient, a 48-hour in-hospital observation period is not required to monitor asymptomatic term infants whose mothers received adequate prophylaxis.36 A 24-hour observation appears sufficient if outpatient criteria are met.22
Neonates who appear to be septic and those who are born to mothers with chorioamnionitis should receive a diagnostic work-up, including blood culture, complete blood count, and possible lumbar puncture before administration of ampicillin and gentamicin is begun (Figure 1).13 Antibiotics should be discontinued after 48 hours if the blood culture is negative.
No universal recommendations exist for the management of the newborn if maternal intrapartum chemoprophylaxis was not given despite an indication.13 However, a single intramuscular dose of aqueous penicillin G (50,000 units per kg within one hour of birth) to newborns whose mothers received less than three hours of antibiotics but did not develop intrapartum risk factors appeared observationally to decrease neonatal sepsis without increasing late-onset (four to 30 days of age) disease, GBS meningitis, or mortality.37