
A more recent article on diagnosis and treatment of melanoma is available.
Am Fam Physician. 2005;72(2):269-276
Author disclosure: Nothing to disclose.
Melanoma is an increasingly common malignancy, and it affects a younger population than most cancers. Risk factors for melanoma include white race, sun sensitivity, family history of melanoma, and melanocytic nevi. Sunburn and intermittent sun exposure appear to increase the risk of developing melanoma. The role of population-based screening for skin cancer remains unclear. Consistent screening results in the diagnosis of thinner melanomas, but there is no evidence that this leads to decreased mortality. The ABCDs—asymmetry, border, color, diameter—can be used as a guide to differentiate melanoma from benign lesions. Suspicious pigmented lesions should undergo full thickness biopsy. Treatment consists of surgical resection, lymph node evaluation, and systemic therapy for some patients. Prognosis depends on the stage at diagnosis. Patients with melanoma require close follow-up because they are at risk for recurrence and diagnosis of a second primary tumor. Preventive strategies for melanoma should emphasize seeking shade when outdoors, wearing protective clothing, and avoiding exposure during the peak sunlight hours.
Skin cancer is the most common malignancy in the United States. Although melanoma represents a small subset, it is the most deadly cutaneous neoplasm. The American Cancer Society estimates that there will be 59,580 new cases of melanoma and 7,770 deaths from melanoma in 2005.1 The incidence of melanoma increases by 4.1 percent per year, faster than any other malignancy.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Physicians may choose to screen patients with a total-body skin examination, especially those at higher risk (evidence insufficient to recommend for or against). | C | 8 |
| Suspicious lesions should undergo full thickness biopsy into the underlying subcutaneous tissue with a 1- to 2-mm border. | C | 11 |
| Shave biopsies should never be used if melanoma is suspected. | C | 11 |
| Surgical resection with wide margins (greater than 3 cm) no longer is recommended. | A | 29,30 |
| Recommended margins are 0.5 cm for melanoma in situ, 1 cm for melanomas with a Breslow thickness of 0.5 to 1 mm, and 2 cm for melanomas with a Breslow thickness greater than 1 mm. | B | 29–31 |
| Nodal evaluation is recommended in patients with melanomas at least 1 mm in thickness. Sentinel lymph node biopsy is recommended in patients with intermediate thickness melanoma (1 to 4 mm) and clinically negative nodes. | B | 33,37,38 |
| Patients with melanoma need intensive follow-up, especially during the first two to three years after diagnosis. | C | 41 |
| Educational and policy changes in elementary schools and similar interventions for adults in recreational settings can increase sun-protective behaviors and are recommended. | C | 5 |
Epidemiology
According to data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, from 1995 to 1999 the annual age-adjusted incidence of melanoma was 20 per 100,000 persons among whites.2 The incidence was higher in men (24.4 per 100,000 persons) than in women (16.8 per 100,000 persons).2 The lifetime risk of developing invasive melanoma is 2.04 percent for white men and 1.45 percent for white women; about one in 74 Americans will be diagnosed with melanoma.2
Melanoma affects a younger patient population than many malignancies. The median age at diagnosis is 57 years. Sixty-two percent of cases are diagnosed before patients reach 65 years of age, and the median age at death is 67 years. Melanoma is the sixth leading cause of cancer death in the United States. According to statistics from SEER, an average of 18.8 life-years are lost per melanoma death.2 Melanoma is much more common in whites than in non-whites. California Cancer Registry data from 1988 to 1993 show the average annual age-adjusted incidence rates per 100,000 persons were 17.2 in men and 11.3 in women for non-Hispanic whites; 2.8 (men) and 3.0 (women) for Hispanics; 0.9 (men) and 0.8 (women) for Asians; and 1.0 (men) and 0.7 (women) for blacks.3
Risk Factors
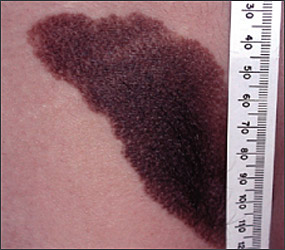
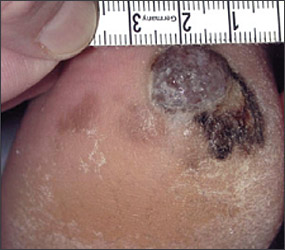
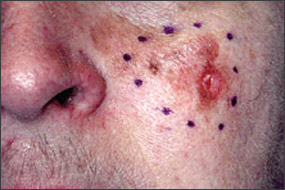
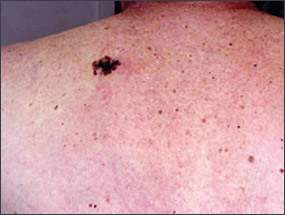
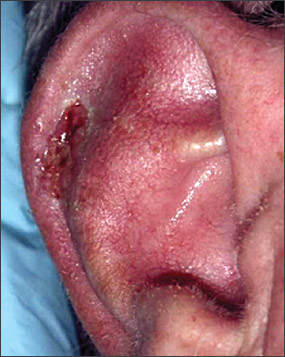
Numerous risk factors for the development of melanoma have been identified, including sun sensitivity, white skin, fair hair, light eyes, tendency to freckle, family history of melanoma, dysplastic nevi, increased numbers of typical nevi, large congenital nevi (Figure 1), and immunosuppression.4 The risk associated with sun exposure is not completely clear. Although sun exposure is a risk factor for melanoma, cutaneous melanomas can arise frequently in areas of the body not exposed to the sun (Figure 2). Sunburn and intermittent sun exposure increase the risk of melanoma, but cumulative and occupational sun exposure do not appear to increase risk.5 Sun exposure in childhood and having more than one blistering sunburn in childhood are associated with an increased risk of melanoma.6
The evidence regarding the use of sunlamps and tanning beds is mixed. About one half of the case-control studies performed revealed an association between sun-lamp or tanning bed use and melanoma, especially if the dose of exposure was high or if it caused skin burning.5
Melanocytic nevi consistently are identified as risk factors for the future development of melanoma. Most melanocytic nevi develop during childhood and adolescence. Sunburn and moderate sun exposure also appear to be related to the development of melanocytic nevi.
In addition to environmental factors, genetic factors influence the risk of melanoma. A family history of melanoma in a first-degree relative is associated with an eight- to 12-fold increase in the risk of melanoma.
Screening
SCREENING BY PHYSICIANS
The U.S. Preventive Services Task Force reviewed the evidence in 2001 and concluded that there is insufficient evidence to recommend for or against routine screening for skin cancer in asymptomatic patients in the primary care clinical setting using a total-body skin examination for the early detection of cutaneous melanoma, basal cell cancer, or squamous cell skin cancer.7,8 Table 14,5,8–18 provides additional screening recommendations.
| U.S. Preventive Services Task Force |
| Insufficient evidence to recommend for or against routine screening for skin cancer in the primary care setting using a total-body skin examination for the early detection of cutaneous melanoma, basal cell carcinoma, or squamous cell carcinoma8 |
| Insufficient evidence to recommend for or against counseling on sun avoidance and use of protective clothing for adults and children at high risk5 |
| Insufficient evidence to recommend for or against sunscreen use5 |
| American Academy of Family Physicians |
| Insufficient evidence to recommend for or against routine skin cancer screening in asymptomatic persons9 |
| Recommends sun avoidance strategies and use of sunscreen with sun protection factor (SPF) of at least 1510 |
| American Academy of Dermatology |
| Recommends that patients at high risk for melanoma should be examined regularly by a dermatologist11 |
| Recommends sun avoidance and covering up with protective clothing4 |
| Recommends sunscreen with SPF of at least 15, covering the entire body, reapplied every two hours4 |
| American College of Preventive Medicine |
| Recommends periodic total cutaneous examinations, targeting populations at high risk for malignant melanoma, defined as persons with family or personal history of skin cancer, predisposing phenotypic characteristics, increased occupational or recreational exposure to sunlight, or clinical evidence of precursor lesions12 |
| National Cancer Institute |
| Insufficient evidence, according to expert opinion, to establish whether a decrease in mortality occurs with routine skin examination14 |
| Insufficient evidence to establish whether other theoretic benefits or harms associated with incorrect diagnosis occur14 |
| Institute of Medicine |
| Insufficient evidence to recommend skin cancer screening; however, physicians and patients must remain alert to the common signs of skin malignancies, with special attention to older white men15 |
| Canadian Task Force on Preventive Health Care |
| Insufficient evidence to recommend for or against skin cancer screening among the general population, but suggests regular total-body skin examinations for persons at high risk16 |
| American Cancer Society |
| Recommends monthly self-examination and physician skin examination as part of a cancer checkup every three years for persons 20 to 40 years of age and annually for persons older than 40 years17 |
| National Institutes of Health |
| Consensus Panel recommends screening for melanoma as part of routine primary care18 |
Selective screening ultimately may be most effective. Compared with the general population, persons with one or two risk factors (e.g., red or blond hair, freckling on the upper back, history of three or more blistering sunburns before 20 years of age, three or more outdoor summer jobs before 20 years of age, actinic keratosis) have 3.5 times the risk of developing melanoma, and in persons with three or more risk factors, the risk is increased 20 times.19 Other predictors of finding a confirmed melanoma during screening include changes in a mole, skin type, personal history of melanoma, and being a middle-aged or older man.20 The development of risk-assessment tools someday may facilitate appropriate selective screening. An unsupervised self-administered questionnaire used in a dermatology setting had moderate usefulness in identifying patients at high risk for melanoma.21 However, at this time there is no validated risk-assessment scale with which to screen patients for melanoma.
Although there is insufficient evidence to recommend routine screening of all patients, physicians should take advantage of the physical examination to screen opportunistically. Lesions that are detected by a physician are significantly thinner (0.40 mm) than those detected by the patient (1.17 mm) or a spouse (1.00 mm; P < .001).22 However, screening studies have not shown a decrease in mortality.
A randomized trial23 of population-based screening for melanoma is underway in Australia. Hopefully, this study will provide a definitive answer to the question of whether skin cancer screening can reduce mortality from melanoma.
SKIN SELF-EXAMINATION
The American Academy of Dermatology and the American Cancer Society provide educational materials for monthly skin self-examination and recommend regular self-examination for everyone. A large case-control study24 showed a decrease in “lethal” melanoma (i.e., death or distant metastases) in persons who performed skin self-examination (measured by interview questionnaire) compared with those who did not.
DIAGNOSIS
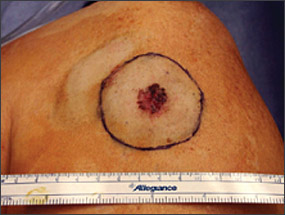
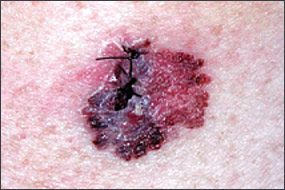
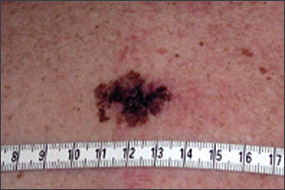
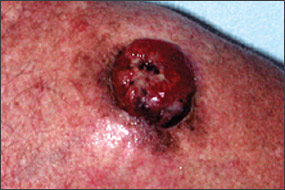
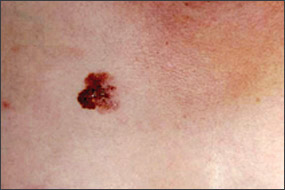
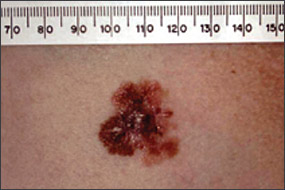
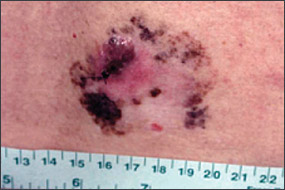
Benign pigmented lesions must be distinguished from early melanoma. The ABCDs (asymmetry, border, color, diameter) of melanoma provide a guide for making this diagnosis.25 Some dermatology experts also add E forevolution or forelevation above skin level. In general, benign lesions are round and symmetric, while melanomas are asymmetric. Benign lesions usually have regular margins, while melanomas have irregular borders. Benign lesions are uniform in color, while melanomas are more heterogeneous, with colors ranging from tan to brown and black, often with areas of red, white, or blue. Finally, most benign lesions are smaller than 6 mm in diameter, while melanomas often are larger than 6 mm at the time of diagnosis (Figures 3 through 10). The ABCD checklist is a sensitive diagnostic test (90 to 100 percent, depending on whether a positive test is defined as the presence of one, two, or three of the ABCDs), but the specificity is not well defined.26 Table 227 describes the histologic subtypes of cutaneous melanoma.
| Type | Frequency (%) | Age at diagnosis | Site | Clinical features |
|---|---|---|---|---|
| Superficial spreading | 70 | Mid 40s | Any | Raised border; brown lesion with pinks, whites, grays, and blues |
| Nodular | 15 | Mid to late 40s | Any | Arises from normal skin or nevus; brown to black lesion |
| Acral lentiginous | 10 | 60s | Hands and feet | Flat, irregular; dark brown to black lesion |
| Lentigo maligna | 5 | 70s | Face | Very irregular border; tan to brown lesion |
Suspicious lesions should undergo full-thickness biopsy into the underlying subcutaneous tissue. Excisional biopsy with 1- to 2-mm borders is preferred. The excisional biopsy should be oriented with the definitive treatment in mind. Incisional or punch biopsies may be performed if lesion size or location makes excisional biopsy inappropriate or impractical. Incisional or punch biopsies should include the area of the lesion that appears most suspicious.11,28 A negative incisional or punch biopsy does not necessarily rule out melanoma in a highly suspicious lesion. Shave biopsies should never be used if melanoma is suspected because lesion thickness is vitally important in determining treatment and prognosis.
Treatment
LOCAL THERAPY
After melanoma is confirmed, patients must undergo complete excision of the tumor or tumor site. Surgical resection is curative for local disease. Randomized controlled trials comparing narrow excision (1 to 2 cm) with wide excision (more than 3 cm) consistently have found equivalent rates of local recurrence and disease-free and overall survival.29,30 Wide excision margins are no longer recommended. Although the exact margins have not been clarified, general recommendations are as follows: melanoma in situ, recommended margin 0.5 cm; melanomas with a Breslow thickness of 1 mm or less, recommended margin 1 cm; and melanomas with a Breslow thickness greater than 1 mm, recommended margin 2 cm.28
NODAL EVALUATION
Patients who are diagnosed with nonulcerated melanoma less than 1 mm deep are unlikely to have nodal metastasis and do not require further surgical evaluation of the lymph nodes. Nodal evaluation is important in patients diagnosed with a melanoma at least 1 mm deep because it determines the overall prognosis and the need for therapeutic lymph node dissection or adjuvant treatment. Historically, the regional lymph node basin was evaluated with an elective lymph node dissection (i.e., removal of all the regional lymph nodes) in patients who did not have clinical evidence of nodal involvement. Elective lymph node dissection no longer is indicated. Local recurrence or overall survival is the same whether patients receive elective lymph node dissection or observation.31
Sentinel lymph node biopsy is a highly accurate diagnostic test to stage the regional nodal basin and has minimal morbidity. This procedure is based on the premise that the primary tumor drains to the regional lymph nodes in a predictable way, and that the first, or sentinel, node can predict accurately the pathologic status of the entire draining nodal basin.32 Sentinel lymph node biopsy has become the standard of care for nodal staging in patients with intermediate thickness melanoma (1 to 4 mm) and clinically negative nodes. Sentinel lymph node biopsy also is feasible in patients who have had a wide local excision in the past.33 Performing sentinel lymph node biopsy requires coordinated expertise between nuclear medicine physicians, pathologists, and surgeons. Preoperative lymphatic mapping is used to accurately identify the draining nodal basin. The sentinel lymph node is examined at multiple levels with hematoxylin and eosin stains, and immunohistochemical stains.
No additional therapy is required for patients with a tumor-free sentinel lymph node. Patients with meta-static tumor in the sentinel lymph node and those who have clinically evident nodal metastasis should undergo therapeutic lymph node dissection of the entire draining nodal basin and a discussion of post-surgical adjuvant therapy options.34
ADJUVANT THERAPY
Although no effective chemotherapy is available for melanoma, interferon alfa-2b (Intron A) is a biologic therapy available for adjuvant treatment of melanoma. Investigators in the initial study35 found a significant increase in relapsefree and overall survival in the group that received high-dose interferon compared with observation alone. Subsequent trials were inconclusive or contradictory. A recent meta-analysis of all available randomized controlled trials found a statistically significant increase in relapsefree survival, but not in overall survival.36 At this time, the use of interferon alfa-2b in patients with melanoma is controversial, particularly in patients with micrometastases in the sentinel node. Clinical trials of other therapies, including combination chemotherapy, vaccines, and hyperthermic isolation limb perfusion, are ongoing.
Prognosis
Survival is influenced strongly by the stage at diagnosis. According to SEER data from 1992 to 1998, 82 percent of melanomas were localized at the time of diagnosis (stage I or II), 9 percent were regional (stage III), and 4 percent had distant metastases (stage IV).2 The overall five-year survival rates were 89 to 96 percent for localized disease, 60 percent for regional disease, and 14 percent for distant disease.2
Important prognostic factors in the primary tumor are tumor thickness and ulceration. Tumors on the head, neck, and trunk have a poorer prognosis, and the rate of disease-specific survival decreases with increasing age at diagnosis. For patients with regional (stage III) disease, the number of metastatic nodes, tumor burden within the nodes, and ulceration of the primary tumor are the most important predictors of survival.37 Evidence shows that patients staged by sentinel lymph node biopsy have improved five-year survival rates compared with patients staged by elective lymph node dissection or clinical examination.38 Another large study39 identified gender and histology as prognostic indicators: men have a lower survival rate than women, and nodular and acral lentiginous spreading indicates a poorer prognosis than lentigo maligna and superficial spreading (Table 2).27
Follow-Up
Compared with the general population, patients diagnosed with melanoma are at significantly increased risk of developing additional melanomas in the future. Most of these second melanomas are diagnosed in the first two years following diagnosis of the first melanoma (mean, 2.8 years; median, one year).40 The one-year, five-year, and 10-year probabilities of developing a second primary tumor are reported to be 1.0, 2.1, and 3.2 percent respectively, more than 10 times the risk in the general population. For this reason, patients diagnosed with melanoma need intensive follow-up, especially during the first two to three years after diagnosis,41 although an optimal follow-up regimen has not been defined.
Prevention
Effective strategies for the prevention of melanoma have been elusive. Evidence-based reviews have found little evidence that physician counseling in the primary care setting is effective in preventing skin cancer.42 A meta-analysis5 found no association between sunscreen use and decreased incidence of melanoma (odds ratio, 1.01). This may be because sunscreens provide a false sense of security and often are used incorrectly, thereby leading to inadequate overall protection from exposure to ultraviolet light.
Evidence shows that some community-level approaches effectively reduce sun exposure. Educational and policy approaches in elementary schools can change sun-protective behavior in children. Similar interventions in recreational and tourism settings can change sun-protective behavior in adults. The Task Force on Community Preventive Services recommends these multicomponent population-based approaches.43 The strongest evidence supports preventing intermittent sunburn in childhood. This is best accomplished by avoiding the midday sun and wearing protective clothing, including long pants, long sleeves, wide-brimmed hats, and sunglasses.5 Sun avoidance should be the cornerstone of preventive strategies.