
Am Fam Physician. 2005;72(2):279-285
A more recent article on fetal alcohol syndrome and fetal alcohol spectrum disorders is available.
Author disclosure: Nothing to disclose.
To complement the 2005 Annual Clinical Focus on medical genomics, AFP will be publishing a series of short reviews on genetic syndromes. This series was designed to increase awareness of these diseases so that family physicians can recognize and diagnose children with these disorders and understand the kind of care they might require in the future. The second review in this series discusses fetal alcohol syndrome and fetal alcohol spectrum disorders.
Fetal alcohol spectrum disorders (FASD) are caused by the effects of maternal alcohol consumption during pregnancy. Fetal alcohol syndrome (FAS) is the most clinically recognizable form of FASD and is characterized by a pattern of minor facial anomalies, prenatal and postnatal growth retardation, and functional or structural central nervous system (CNS) abnormalities. The consequences are lifelong, and the behavioral and learning difficulties are often greater than the degree of neurocognitive impairment. Alcoholrelated neurodevelopmental disorder also is a clinically recognizable diagnosis in the continuum of FASD and describes the clinical outcome when the facial features typical of FAS are absent.
Epidemiology
FASD may affect up to 1 percent of the U.S. population.1,2 Birth defects related to these disorders can be prevented by avoiding alcohol ingestion during pregnancy. Although FASD is more strongly associated with higher levels of alcohol consumption compared with lower levels, animal studies have suggested that even a single episode of consuming the equivalent of two alcoholic drinks during pregnancy may lead to loss of fetal brain cells (one drink = 12 oz of beer, 5 oz of wine, or 1.5 oz of “hard” liquor).3 Despite widespread knowledge of alcohol’s deleterious effects, a study4 of women between 18 and 44 years of age showed that 10 percent used alcohol during pregnancy and that 2 percent engaged in “binge drinking” (i.e., five or more drinks on one occasion). Maternal factors that increase the risk of FASD include being older than 30 years, a history of binge drinking, and low socioeconomic status.5
Clinical Presentation
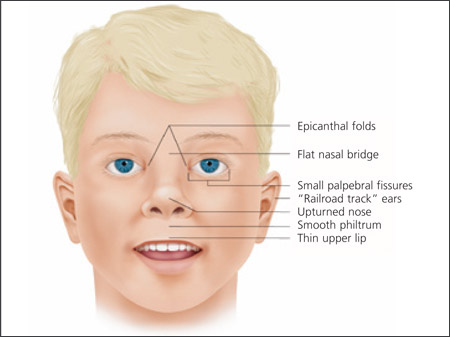
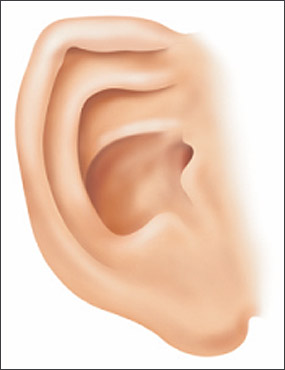
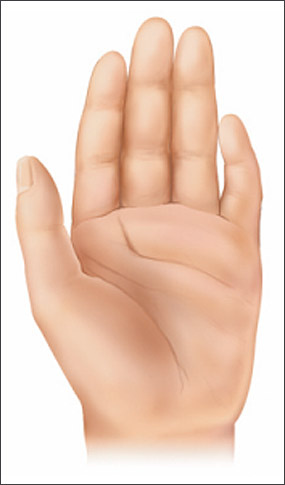
Clinical findings in a newborn with FAS include a characteristic pattern of facial anomalies,6,7 with short palpebral fissures, a thin upper lip, and a long, smooth philtrum (Figures 1, 2a through 2d, and 3a through 3c).4,8 Other findings may include a flat midface, ptosis of the eyelids, epicanthal folds, an upturned nose with a flat nasal bridge, underdeveloped ears (“railroad track” appearance, Figure 4), clinodactyly of the fifth fingers (Figure 5), camptodactyly, “hockey stick” palmar creases (Figure 5), hirsutism, and cardiac defects. Prenatal or postnatal growth retardation typically results in a height or weight below the 10th percentile for age and race. Microcephaly is common, as are structural brain anomalies. CNS impairment may not be apparent in newborns. Cognitive deficits and behavioral anomalies such as attention-deficit/hyperactivity disorder typically become more apparent in school-aged children with FASD and usually persist into adolescence and adulthood, whereas facial findings among the subset with FAS may become less characteristic.
Diagnosis
Diagnosis of FASD should be considered based on the clinical presentation or suspicion of maternal alcohol exposure. The Centers for Disease Control and Prevention diagnostic criteria for FAS require three specific facial findings (i.e., smooth philtrum, thin vermilion border of the upper lip, and short palpebral fissures), growth deficits, and CNS abnormalities (Table 14 and Figure 3). In the absence of characteristic facial findings, the diagnosis of FASD still should be considered in children with growth problems, CNS abnormalities, and a history of prenatal alcohol exposure.
| Clinical feature | Criteria |
|---|---|
| Shortened palpebral fissures | ≤10th percentile for age and racial norms |
| Smooth philtrum | Score of 4 or 5 on Lip-Philtrum Guide (Figure 3) |
| Thin vermilion border of upper lip | Score of 4 or 5 on Lip-Philtrum Guide |
Because of the associated guilt or stigma, direct questioning or commonly used screening questionnaires such as the four-item CAGE criteria9 (Cut down, Annoyance, Guilt, Eye-opener) may fail to identify women at risk for delivering a child with FASD. One study10 showed the T-ACE (Tolerance, Annoyance, Cut down, Eye-opener) assessment to be more effective than other screening tools (Table 210,11).
| Tolerance: How many drinks does it take to make you feel high? |
| Annoyance: Have people annoyed you by criticizing your drinking? |
| Cut down: Have you ever felt you ought to cut down on your drinking? |
| Eye-opener: Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover? |
| Score 2 points for a response of at least six drinks (either beer or mixed drinks) or one bottle of wine to the first question; 1 point is given for a positive response to the other questions. A score of 2 or more identifies 90 percent of women at risk for alcohol misuse. |
Causes of Fetal Alcohol Spectrum Disorders
FASD is nonhereditary; alcohol causes neuronal damage and cell loss in the fetal brain through direct action as a toxin. No prenatal period has been shown to be safe from the deleterious effects of alcohol. CNS damage may result from alcohol exposure in any trimester, even before the time of a pregnancy test. Women should be advised not to drink from the time of conception to birth.
Although FASD is not inherited, there seems to be a genetic predisposition to problem drinking. For example, in some persons of shared ancestry, such as Native Americans, the similarity in function of shared versions (alleles) of the gene that encodes alcohol dehydrogenase may contribute to an increased risk for alcohol dependence.12 Additionally, twin studies document similar outcomes in identical twins and different outcomes in nonidentical twins.6
Referral and Management
Referral to a multidisciplinary team (i.e., dysmorphologist/clinical geneticist, developmental pediatrician, mental health professional, social worker, and educational specialist) often is necessary for diagnosis because the growth, CNS, and facial features of FASD can overlap other syndromes and disorders. Even in patients who clearly have met diagnostic criteria, referral allows complete assessment for an individualized management plan (Table 34).
| Known prenatal alcohol exposure | ||
| Maternal alcohol exposure of 7 or more drinks per week or 3 or more drinks on multiple occasions | ||
| Unknown prenatal alcohol exposure | ||
| Caregiver or parental concern | ||
| Presence of the following facial features: | ||
| Smooth philtrum | ||
| Thin vermilion border of the upper lip | ||
| Small palpebral fissures | ||
| or | ||
| Presence of one or more facial features in addition to deficit in height or weight | ||
| or | ||
| Presence of one or more facial features in addition to central nervous system abnormalities | ||
Treatment involves coordination of multiple community services. Social services are needed to ensure a safe home environment and provide parental education. Educational support often is needed most. Early identification and intervention results in significantly improved outcomes. A diagnosis of FAS meets the “presumptive diagnosis” requirements of Part C of the Individuals with Disabilities Education Act, allowing children younger than three years to receive services even if their test results fall in the normal range. After three years of age, eligibility for services often depends on demonstration of a specific functional deficit.
Genomics Glossary
Camptodactyly: Permanent flexion contracture of a finger or toe.
Clinodactyly: Permanent curving of the fifth finger, usually toward the other fingers; also seen for other fingers or toes.
Epicanthal folds: Skinfolds covering the inner corners of the eyes. Epicanthal folds occur commonly in infants and may persist in persons of certain ancestries.
“Hockey stick” palmar crease: Transverse flexion crease of the palm close to the fingers; widens like the end of a hockey stick and ends between the second and third fingers.
Palpebral fissure length: Distance between the inner and outer corners of one eye.
Philtrum: Vertical groove between the nose and upper lip; a flat or smooth philtrum can present in persons with fetal alcohol spectrum disorders.
Ptosis: Drooping of the upper eyelids.
“Railroad track” ears: The top part (curve) of the outer ear is underdeveloped, folded over, and parallel to the curve beneath it, giving the appearance of a railroad track.
Vermilion border: Edge of the lip where it meets the skin of the face
Resources
Additional information about FASD and FAS is available online at the following Web sites:
• Substance Abuse and Mental Health Services Administration:http://fascenter.samhsa.gov
• National Center on Birth Defects and Developmental Disabilities:http://www.cdc.gov/ncbddd/fas
• Fetal Alcohol Syndrome Diagnostic and Prevention Network:http://depts.washington.edu/fasdpn
• National Organization on Fetal Alcohol Syndrome:http://www.nofas.org.