
A more recent article on diagnosis and management of rheumatoid arthritis is available.
Am Fam Physician. 2005;72(6):1037-1047
Patient information: See related handout on rheumatoid arthritis, written by the authors of this article.
Author disclosure: Nothing to disclose
Rheumatoid arthritis is a chronic inflammatory disease characterized by uncontrolled proliferation of synovial tissue and a wide array of multisystem comorbidities. Prevalence is estimated to be 0.8 percent worldwide, with women twice as likely to develop the disease as men. Untreated, 20 to 30 percent of persons with rheumatoid arthritis become permanently work-disabled within two to three years of diagnosis. Genetic and environmental factors play a role in pathogenesis. Although laboratory testing and imaging studies can help confirm the diagnosis and track disease progress, rheumatoid arthritis primarily is a clinical diagnosis and no single laboratory test is diagnostic. Complications of rheumatoid arthritis may begin to develop within months of presentation; therefore, early referral to or consultation with a rheumatologist for initiation of treatment with disease-modifying antirheumatic drugs is recommended. Several promising new disease-modifying drugs recently have become available, including leflunomide, tumor necrosis factor inhibitors, and anakinra. Nonsteroidal anti-inflammatory drugs, corticosteroids, and nonpharmacologic modalities also are useful. Patients who do not respond well to a single disease-modifying drug may be candidates for combination therapy. Rheumatoid arthritis is a lifelong disease, although patients can go into remission. Physicians must be aware of common comorbidities. Progression of rheumatoid arthritis is monitored according to American College of Rheumatology criteria based on changes in specific symptoms and laboratory findings. Predictors of poor outcomes in early stages of rheumatoid arthritis include low functional score early in the disease, lower socioeconomic status, early involvement of many joints, high erythrocyte sedimentation rate or Creactive protein level at disease onset, positive rheumatoid factor, and early radiologic changes.
Rheumatoid arthritis is characterized by persistent joint synovial tissue inflammation. Over time, bone erosion, destruction of cartilage, and complete loss of joint integrity can occur. Eventually, multiple organ systems may be affected.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Patients with rheumatoid arthritis should be treated as early as possible with DMARDs to control symptoms and delay disease progression. | A | 2,14,15 |
| Patients with persistent inflammatory joint disease (longer than six to eight weeks) already receiving analgesics or NSAIDs should be considered for rheumatology referral, preferably within 12 weeks. | C | 13,14 |
| Patient education, preferably one-to-one, should be provided when rheumatoid arthritis is diagnosed. | C | 29 |
| NSAIDs should be prescribed in the lowest dose that provides symptom relief and should be cut back after a good response to DMARDs is achieved. | A | 13 |
| Gastroprotection should be used if patients are older than 65 years or have a history of pepticulcer disease. | B | 13 |
| Intra-articular corticosteroid injections can be helpful but should not be administered more than three times in one year. | C | 13 |
| Low-dose oral corticosteroids are effective for symptom relief but have a high risk of toxicity; therefore, the lowest dosage possible should be used for the shortest period possible. | A | 2 |
| Combination therapy may be more effective than treatment with one drug alone. | A | 2,16–18 |
| Efficacy of treatment should be monitored; changes in hemoglobin, erythrocyte sedimentation rate, and C-reactive protein may indicate treatment response, and measurement instruments such as the European League Against Rheumatism response criteria are useful for tracking disease progression. | C | 2,35 |
| A multidisciplinary team approach is beneficial, at least in the short term; therefore, patients should have access to a wide range of health care professionals, including their primary care physicians, rheumatologists, nursing specialists, physical therapists, occupational therapists, dietitians, podiatrists, pharmacists, and social workers. | C | 30 |
| Exercise is beneficial for aerobic capacity and muscle strength with no detrimental effects on disease activity or pain levels. | C | 28 |
Rheumatoid arthritis is the most common inflammatory arthritis, affecting 0.8 percent of the adult population worldwide. Onset usually occurs between 30 and 50 years of age. Incidence in the United States is estimated as 25 per 100,000 persons for men and 54 per 100,000 persons for women.1 Rheumatoid arthritis is responsible for an estimated 250,000 hospitalizations and 9 million physician visits each year.2 Its economic impact is magnified by the high level of functional impairment it causes: untreated, 20 to 30 percent of persons with rheumatoid arthritis become permanently work-disabled within three years of diagnosis.3
Etiology and Pathophysiology
The etiology of rheumatoid arthritis is not fully understood. Evidence points to a complex interplay between environmental and genetic factors. In monozygotic twins, there is a more than 30 percent concordance rate for rheumatoid arthritis development, and 80 percent of whites with rheumatoid arthritis express the HLA-DR1 or -DR4 subtypes. These and other regions of the Major Histocompatibility Complex may confer susceptibility to more severe disease by causing a specific arthrogenic peptide to be presented to CD4+ T cells.4
Joint damage in rheumatoid arthritis begins with the proliferation of synovial macrophages and fibroblasts after a triggering incident, possibly autoimmune or infectious. Lymphocytes infiltrate perivascular regions, and endothelial cells proliferate. Neovascularization then occurs. Blood vessels in the affected joint become occluded with small clots or inflammatory cells. Over time, inflamed synovial tissue begins to grow irregularly, forming invasive pannus tissue. Pannus invades and destroys cartilage and bone. Multiple cytokines, interleukins, proteinases, and growth factors are released, causing further joint destruction and the development of systemic complications.1,4
RISK FACTORS
Female sex, a positive family history, older age, silicate exposure, and smoking are associated with an increased risk for developing rheumatoid arthritis.1,5,6 Consumption of more than three cups of coffee daily—particularly decaffeinated coffee—also may contribute.7 High vitamin D intake,8 tea consumption,7 and oral contraceptive use6 are associated with decreased risk. Three in four women with rheumatoid arthritis experience significant improvement in symptoms when pregnant, usually with a recurrence after delivery.5 A systematic review9 of 24 studies did not support a link between breast implants and connective tissue disorders.
Diagnosis
TYPICAL PRESENTATION
Rheumatoid arthritis primarily is a clinical diagnosis. Patients commonly present with pain and stiffness in multiple joints, although one third of patients initially experience symptoms at just one location or a few scattered sites. In most patients, symptoms emerge over weeks to months, starting with one joint and often accompanied by prodromal symptoms of anorexia, weakness, or fatigue. In approximately 15 percent of patients, onset occurs more rapidly over days to weeks. In 8 to 15 percent of patients, symptoms begin within a few days of a specific inciting event, such as an infectious illness.5
Joints most commonly affected are those with the highest ratio of synovium to articular cartilage. The wrists are nearly always involved, as are the proximal interphalangeal and metacarpophalangeal joints. The distal interphalangeal joints and sacroiliac joints tend not to be affected.5 Rheumatoid joints typically are boggy, tender to the touch, and warm, but they usually are not erythematous. Some patients complain of “puffy” hands secondary to increased blood flow to inflamed areas. Prominent epitrochlear, axillary, and cervical lymph nodes may be noted. Muscles near inflamed joints often atrophy. Weakness is commonly out of proportion to pain on examination. Morning stiffness lasting at least 45 minutes after initiating movement is common. Patients often hold joints in flexion to minimize painful distension of joint capsules. Low-grade fever, fatigue, malaise, and other systemic complaints may arise, especially in an acute presentation.5,10
DIAGNOSTIC CRITERIA
In clinical trials, rheumatoid arthritis is diagnosed formally using seven American Rheumatism Association (ARA) criteria (Table 1).11,12 In typical outpatient practice, a definitive diagnosis using these criteria may be difficult to obtain early in the disease process. During the initial visit, patients should be asked about degree of pain, duration of stiffness and fatigue, and functional limitations. A careful joint examination looking for the characteristics described above is vital.
| Sign or symptom | Definition | LR+ | LR- | Percentage with rheumatoid arthritis if sign or symptom is*: | |
|---|---|---|---|---|---|
| Present | Absent | ||||
| Morning stiffness | Stiffness in or around the affected joints for at least one hour after initiating movement | 1.9 | 0.5 | 39 | 14 |
| Arthritis of three or more joint areas | Three or more of the following joints noted to be fluid-filled or have soft tissue swelling: wrist, PIP, MCP, elbow, knee, ankle, MTP | 1.4 | 0.5 | 32 | 13 |
| Hand joint involvement | Wrist, MCP, or PIP joints among the symptomatic joints observed | 1.5 | 0.4 | 33 | 12 |
| Symmetric arthritis | Right and left joints involved for one or more of following: wrist, PIP, MCP, elbow, knee, ankle, MTP† | 1.2 | 0.6 | 29 | 17 |
| Rheumatoid nodules | Subcutaneous nodules in regions surrounding joints, extensor surfaces, or bony prominences | 3.0 | 0.98 | 50 | 25 |
| Serum rheumatoid factor positive | Positive result using any laboratory test that has a positive predictive value of 95 percent or more (i.e., is positive in no more than 5 percent of patients without rheumatoid arthritis) | 8.4 | 0.4 | 74 | 13 |
| Radiographic changes | Hand and wrist films show typical changes of erosions or loss of density adjacent to affected joints | 11 | 0.8 | 79 | 21 |
DIFFERENTIAL DIAGNOSIS
Rheumatoid arthritis must be differentiated from a number of other disorders. Infection-related reactive arthropathies, seronegative spondyloarthropathies, and other connective tissue diseases such as systemic lupus erythematosus may have symptoms in common with rheumatoid arthritis, as may an array of endocrine and other disorders (Table 2).5,10 Gout rarely coexists with rheumatoid arthritis, and a joint aspiration should be considered if gout is suspected.
| Diagnosis | Comments |
|---|---|
| Connective tissue diseases | Such as scleroderma and lupus |
| Fibromyalgia | Evaluate for trigger points. |
| Hemochromatosis | Iron studies and skin coloration changes may guide diagnosis. |
| Infectious endocarditis | Rule out murmurs, high fever, and history of intravenous drug use. |
| Polyarticular gout | Joints often erythematous; podagra commonly found; gout and rheumatoid arthritis rarely coexist, but calcium pyrophosphate deposition disease can accompany rheumatoid arthritis. |
| Polymyalgia rheumatica | Rheumatoid arthritis, unlike polymyalgia rheumatica, rarely presents with pain in the proximal joints of the extremities only. |
| Sarcoidosis | Granulomas likely, as are hypercalcemia and chest film findings. |
| Seronegative spondyloarthropathies, reactive arthritis | Tend to be more asymmetric than rheumatoid arthritis. More commonly involve the joints of the spine. Evaluate for history of psoriasis, Reiter’s comorbidities, inflammatory bowel disease. Reactive arthritis can be postinfective, sexually acquired, or related to gastrointestinal disorders. |
| Still’s disease | Tends to present with fever, leukocytosis with left shift, sore throat, splenomegaly, liver dysfunction, and/or rash. |
| Thyroid disease | Consider thyroid-stimulating hormone level depending on symptoms. |
| Viral arthritis | Consider parvovirus, hepatitis B. |
DIAGNOSTIC TESTS
No single diagnostic test definitively confirms the diagnosis of rheumatoid arthritis. However, several tests can provide objective data that increase diagnostic certainty and allow disease progression to be followed. The American College of Rheumatology Subcommittee on Rheumatoid Arthritis (ACRSRA) recommends that baseline laboratory evaluations include a complete blood cell count with differential, rheumatoid factor, and erythrocyte sedimentation rate (ESR) or Creactive protein (CRP). Baseline evaluation of renal and hepatic function also is recommended because these findings will guide medication choices.2 Table 32,5,13 summarizes the test findings associated with rheumatoid arthritis.
| Laboratory test | Associated findings |
|---|---|
| Creactive protein* | Typically increased to >0.7 picograms per mL; may be used to monitor disease course. |
| Erythrocyte sedimentation rate* | Often increased to >30 mm per hour; may be used to monitor disease course. |
| Hemoglobin/hematocrit* | Slightly decreased; hemoglobin averages around 10 g per dL (100 g per L); normochromic anemia, also may be normocytic or microcytic. |
| Liver function* | Normal or slightly elevated alkaline phosphatase |
| Platelets* | Usually increased |
| Radiographic findings of involved joints* | May be normal or show osteopenia or erosions near joint spaces in early disease; wrist and ankle films are useful as baselines for comparison with future studies. |
| Rheumatoid factor* | Negative in 30 percent of patients early in illness; if initially negative, can repeat six to 12 months after disease onset; can be positive in numerous other processes (e.g., lupus; scleroderma; Sjögren’s syndrome; neoplastic disease; sarcoidosis; various viral, parasitic, or bacterial infections); not an accurate measure of disease progression. |
| White blood count* | May be increased |
| Anticyclic citrullinated peptide antibody | Tends to correlate well with disease progression; increases sensitivity when used in combination with rheumatoid factor; more specific than rheumatoid factor (90 versus 80 percent); not readily available in many laboratories. |
| Antinuclear antibody | Limited value as a screening study for rheumatoid arthritis |
| Complement levels | Normal or elevated |
| Immunoglobulins | Elevated alpha-1 and alpha-2 globulins possible. |
| Joint fluid evaluation | Consider if an affected joint can be tapped and diagnosis is uncertain; straw-colored fluid with fibrin flecks often seen; fluid may clot at room temperature; 5,000 to 25,000 white blood cells per mm3 (5 to 25 × 109 per L) with 85 percent polymorphonuclear leukocytes a common finding; in rheumatoid arthritis, cultures are negative, there are no crystals, and fluid glucose level typically is low. |
| Urinalysis | Microscopic hematuria or proteinuria may be present in many connective tissue diseases. |
Treatment
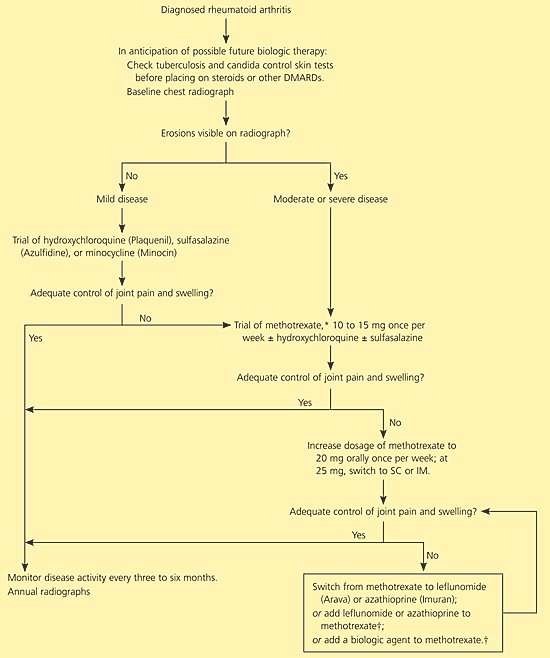
Joint destruction in rheumatoid arthritis begins within a few weeks of symptom onset; early treatment decreases the rate of disease progression.14 Therefore, it is imperative to diagnose the disease and initiate treatment as soon as possible. The ACRSRA recommends that patients with suspected rheumatoid arthritis be referred within three months of presentation for confirmation of diagnosis and initiation of treatment with disease-modifying antirheumatic drugs (DMARDs).2 Therapeutic goals include preservation of function and quality of life, minimization of pain and inflammation, joint protection, and control of systemic complications.2,5 Figure 12,4 outlines an approach to the treatment of a patient with rheumatoid arthritis.
PHARMACOTHERAPY
Pharmacotherapy for rheumatoid arthritis generally involves a nonsteroidal anti-inflammatory drug (NSAID) for control of pain, with selective use of low-dose oral or intra-articular glucocorticoids, and initiation of a DMARD. Other analgesics also may be used, but details are outside the scope of this article. In past decades, pharmacologic treatment of rheumatoid arthritis was managed using a pyramid approach: symptom-alleviating treatment was started at diagnosis, and only with progression of symptoms were dosages changed or additional medications added. However, a “reverse pyramid” approach now is favored, in which DMARDs are initiated quickly to slow disease progression as early as possible (Figure 12,4).15 This change of approach is a result of several research findings: (1) joint damage begins early in the disease14; (2) DMARDs have significant benefits when used early; (3) the benefits of DMARDs may be enhanced when the drugs are used in combination16–18; (4) a number of new DMARDs are available, with good evidence of beneficial effect.19
Patients with mild disease and normal radiographic findings can begin treatment with hydroxychloroquine (Plaquenil), sulfasalazine (Azulfidine), or minocycline (Minocin), although methotrexate also is an option. Patients with more severe disease or radiographic changes should begin treatment with methotrexate. If symptoms are not adequately controlled, leflunomide (Arava), azathioprine (Imuran), or combination therapy (methotrexate plus one of the newer agents) may be considered. Individual drug categories are discussed below.
NSAIDs
NSAIDs, salicylates, or cyclooxygenase-2 inhibitors are used for initial treatment of rheumatoid arthritis to reduce joint pain and swelling. However, because they do not alter the disease course, they should not be used alone.2 Patients with rheumatoid arthritis are almost two times more likely to have serious complications from NSAID use than patients with osteoarthritis, and they should be observed closely for symptoms of gastrointestinal side effects.2,13 Cyclooxygenase-2 inhibitors must be used with caution, given recent findings regarding potential adverse effects.
Glucocorticoids
Steroids at dosages equivalent to less than 10 mg of prednisone daily are highly effective for relieving symptoms of rheumatoid arthritis2 and can slow joint damage.20 Steroid dosages should be kept at a minimum because of the high risk of side effects, which include osteoporosis, cataracts, cushingoid symptoms, and abnormalities in blood glucose levels. The American College of Rheumatology (ACR) guidelines2 recommend that patients being treated with glucocorticoids take 1,500 mg of calcium and 400 to 800 IU of vitamin D daily. When a single inflamed joint contributes to significant disability, injection of glucocorticoids is a safe and effective intervention; however, the effects are temporary. Infectious arthritis should be ruled out before injections are performed.2 Symptoms may recur with steroid discontinuation, especially when high dosages were used, and most rheumatologists withdraw steroids slowly, over a month or more, to avoid rebound effects.21 Systemic steroids often are used as “bridging therapy” during the period when DMARDs have been initiated but have not yet taken effect, but some of the newer DMARDs have a relatively rapid onset of action.2
DMARDs
DMARDs should be considered for all patients with rheumatoid arthritis. Compliance, disease severity, physician experience, and the presence of various comorbidities guide medication choice. The most commonly used medications are methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, infliximab (Remicade), and etanercept (Enbrel).2 Sulfasalazine or hydroxychloroquine often are started first, but in more severe cases, methotrexate or combination therapy may be first-line treatment. Increasing evidence indicates that combinations of DMARDs can be more effective than single-drug regimens. Women of child-bearing age should use adequate contraception when taking certain DMARDs because they could be harmful to a fetus.19 Table 42,13,19 lists DMARD dosing regimens, costs, times to onset of benefit, and adverse effects.
| DMARD | Dosage | Cost (generic)* | Time to benefit | Adverse effects | Monitoring† | Comments | |
|---|---|---|---|---|---|---|---|
| Adalimumab (Humira) | 40 mg SC every two weeks | $1,316 | A few days to four months‡ | Infusion reactions; increased infection risk, including TB reactivation | Monitor for TB, histoplasmosis, and other infections; CBC and ALT at baseline and monthly until dose is stable; may continue every two to three months thereafter. | Monoclonal antibody to TNF-3; shown to reduce disease activity with acceptable safety. | |
| Rare: demyelinating disorders | |||||||
| Anakinra (Kineret) | 100 to 150 mg SCper day | 1,251 | Within 12 weeks; lasting effects by 24 weeks | Infections and decreased neutrophil counts; headaches, dizziness; nausea | CBC at baseline, monthly for three months, then every three months | Interleukin-1 receptor antagonist; used when treatment with another DMARD has failed. | |
| Rare: hypersensitivity | |||||||
| Auranofin (Ridaura) | 3 mg orally twice per day or 6 mg orally per day | 215 | Four to six months | Diarrhea | CBC and urine protein (by dipstick) every one to three months | Has modest effects compared with other DMARDs. | |
| Rare: leukopenia | |||||||
| Azathioprine (Imuran) | 50 to 150 mg orally per day | 76 (39 to 40) | Two to three months | Nausea | CBC every one to two weeks until dose is stable, then every one to three months | Has greater toxicity and is used less commonly than other DMARDs. | |
| Rare: leukopenia; sepsis; lymphoma | |||||||
| Cyclosporine (Gengraf, Neoral, generic) | 2.5 to 5 mg per kgorally per day | 288 to 231 (288 to 326) | Two to four months | Nausea; paresthesias, tremor; headaches; gingival hypertrophy; hypertrichosis | Creatinine every two weeks until dose is stable, then monthly; consider CBC, LFTs, and potassium level tests | Significant clinical benefit up to one year; adverse effects limit use. | |
| Rare: hypertension; renal disease; sepsis | |||||||
| D-Penicillamine (Cuprimine) | 250 to 750 mg orally per day | 35 to 95 | Three to six months | Nausea; loss of taste; rash; reversible platelet decrease | CBC and urinary protein by dipstick every two weeks until dose is stable, then every one to three months | Used less commonly than other DMARDs. | |
| Rare: proteinuria; late autoimmune disease | |||||||
| Etanercept (Enbrel) | 25 mg SC twice per week or 50 mg SC per week | 1,316 | A few days to 12 weeks | Contraindicated in infection; mild injection site reactions | CBC and ALT at baseline and monthly until dose is stable; may continue every two to three months thereafter. | Combination of TNF receptor and portion of IgG1; inhibits TNF-3; slows joint damage. | |
| Rare: demyelination | |||||||
| Hydroxychloroquine (Plaquenil) | 200 to 400 mg orally per day | 57 (33 to 37) | Two to six months | Nausea; headaches | Eye examinations every 12 months in patients older than 40 years and those with previous eye disease | Can be used when diagnosis uncertain; moderate effect but relatively low toxicity. | |
| Rare: abdominal pain; myopathy; retinal toxicity | |||||||
IM gold
| 25 to 50 mg IM every two to four weeks | 34 | Six to eight weeks | Mouth ulcers; rash; vasomotor symptoms after injection | CBC and urinary protein by dipstick every two weeks until dose is stable, then with each injection | Has significant withdrawal rate in trials because of toxicity. | |
| Rare: leukopenia; thrombocytopenia; proteinuria; colitis | |||||||
| Infliximab (Remicade) | 3 mg per kg IV at weeks zero, 2, and 6, then every eight weeks§ | 1,383 (for eight weeks) | A few days to four months‡ | Infusion reactions; increased infection risk, including TB reactivation | Monitor for TB, histoplasmosis, and other infections; CBC and ALT at baseline and monthly until dose is stable; may continue every two to three months thereafter. | Monoclonal antibody to TNF-3; reduces disease activity with acceptable safety. | |
| Rare: demyelinating disorders | |||||||
| Leflunomide (Arava) | 100 mg orally per day for three days, then 10 to 20 mg orally per day | 372 | Four to 12 weeks (tending toward four) | Nausea, diarrhea; rash; alopecia; highly teratogenic, even after discontinuation | Hepatitis B and C serology in high-risk patients; CBC, creatinine, and LFTs monthly for six months, then every one to two months; repeat AST or ALT in two to four weeks if initially elevated, and adjust dose as needed. | Inhibits pyrimidine synthesis and may suppress T-cell activation; improves multiple clinical outcomes and delays radiographic changes; can be eliminated from system with cholestyramine in patients wishing to conceive. | |
| Rare: leukopenia; hepatitis; thrombocytopenia | |||||||
| Methotrexate | 12 to 25 mg orally, IM, or SC per week | Oral: 79 (57 to 65) IM or SC: (18 to 20) | One to two months | Nausea, diarrhea; fatigue; mouth ulcers; rash, alopecia; abnormal LFTs | CBC, creatinine, and LFTs monthly for six months, then every one to two months; repeat AST or ALT in two to four weeks if initially elevated, and adjust dose as needed; liver biopsy if no resolution on discontinuation. | Rapid onset (six to 10 weeks); tends to produce more sustained results over time than other DMARDs and lowers all-cause mortality; can be used when cause of polyarthritis uncertain; often combined with newer DMARDs. | |
| Rare: low WBC and platelets; pneumonitis; sepsis; liver disease; Epstein-Barr virus–related lymphoma; nodulosis | |||||||
| Minocycline (Minocin) | 100 mg orally twice per day | 231 (115 to 147) | One to three months | Dizziness; skin pigmentation | None needed | Effective in combination with prednisone for management of new-onset rheumatoid arthritis. | |
| Staphylococcal protein A immunoadsorption (Prosorba column) | Extracorporeal; weekly for 12 weeks | 20,433 | Three months | Hypotension and anemia during procedure; catheter site infection, joint pain, fatigue | Follow CBC | Used only in refractory patients when many other treatments have failed. | |
| Sulfasalazine (Azulfidine) | 2 to 3 g orally per day in divided doses | 48 (14 to 31) | One to three months | Nausea, diarrhea; headache; mouth ulcers; rash, alopecia; contact lens staining; reversible oligospermia; abnormal LFTs | CBC every two to four weeks for three months, then every three months | Rapid onset (eight to 13 weeks); enteric, coated forms available; can be used when diagnosis uncertain; modest effects compared with other medications. | |
| Rare: leukopenia | |||||||
Newer DMARDs
Several new drugs with novel mechanisms of action have emerged in recent years, including leflunomide, tumor necrosis factor (TNF) antagonists, and anakinra (Kineret).19 Leflunomide is a competitive inhibitor of an intracellular enzyme needed for de novo pyrimidine synthesis by activated lymphocytes.19 Leflunomide slows progression of joint damage as measured radiographically, and it was found to prevent new joint erosions in 80 percent of patients over a two-year period.22
TNF antagonists lower the levels of TNF-3, which is present in increased concentrations in the synovial fluid in patients with rheumatoid arthritis. Etanercept is a soluble TNF-receptor fusion protein. Its long-term effects are comparable with methotrexate in some studies,13,23 but it elicits improvement in symptoms much more rapidly, often within two weeks.23 Infliximab, another TNF antagonist, is a chimeric IgG1 anti–TNF-α antibody. Patients who had a poor response to methotrexate had a greater response with infliximab than placebo (52 versus 17 percent, number needed to treat = 3).17 Adalimumab (Humira) also is a recombinant human IgG1 antibody and has an additive effect when taken with methotrexate.18 TNF antagonists are associated with an increased risk of infection, especially tuberculosis reactivation.19
Anakinra is a recombinant interleukin-1 receptor antagonist. Several randomized controlled trials19,24 have found it to be more effective than placebo when administered alone or in combination with methotrexate. Adverse effects include skin irritation at the site of injection, increased risk of infection, and leukopenia.19
Other new DMARDs are in development. Rituximab (Rituxan), an antibody to a surface receptor on B cells, has shown promise in preliminary studies. Anti–interleukin-6 receptor antibodies also are being evaluated.25
ADJUNCTIVE THERAPIES
A number of additional, nonpharmacologic treatments for rheumatoid arthritis have been tried. Therapeutic fasting, dietary supplementation of essential fatty acids, and journaling have shown benefit,26 as have spa therapies27 and exercise.28 Patient education29 and a multi-disciplinary approach to patient care30 provide at least short-term benefits. Evidence is inconclusive regarding herbal medications,31 acupuncture,26 and splinting.32 Surgery should be considered when pain is unacceptable, loss of motion is significant, or functional impairment is severe.2 More information about nonmedical therapies is available in the online version of this article (https://www.aafp.org/afp/20050915/1037.html).
DURATION OF TREATMENT
Rheumatoid arthritis is a lifelong illness. Combinations of methotrexate and the new biologic agents can lead to remission in 30 to 40 percent of patients with rheumatoid arthritis, but for most patients, significant disease persists despite treatment.16,19 Complete remission rarely occurs. In clinical trials, improvement has been tracked using the ACR improvement criteria, most often ACR 20, ACR 50, or ACR 70. The numbers represent the percentage of improvement in the following criteria: number of tender joints, number of swollen joints, global disease activity (as assessed by the patient or by an observer), pain level, physical disability score, and acute phase response (as measured by CRP or ESR).33 For the individual patient, health assessment questionnaires may be a more useful means of evaluating disease progression. Examples include the European League Against Rheumatism response criteria for rheumatoid arthritis and various daily activity score surveys.2,13 Radiologic assessment scales also are useful.2,34 Treatment should be guided by individual clinical response to various interventions. Changes in hemoglobin, ESR, and CRP may serve as helpful indicators of response to treatment, but platelet count and rheumatoid factor levels tend not to correlate well.13
COMPLICATIONS
| Complication | Comments |
|---|---|
| Anemia | Correlates with erythrocyte sedimentation rate and disease activity; three fourths of patients have anemia of chronic disease; one fourth of patients respond to iron therapy. |
| Cancer | May be secondary to treatments; lymphomas and leukemias two to three times more common in patients with rheumatoid arthritis; increased risk for various solid tumors; genitourinary cancer risk is reduced in rheumatoid arthritis, perhaps because of nonsteroidal anti-inflammatory drugs. |
| Cardiac complications | Pericarditis—one third of patients may have asymptomatic pericardial effusion at diagnosis; atrioventricular block—rare; myocarditis—diffuse inflammation can occur, may or may not be symptomatic. |
| Cervical spine disease | Tenosynovitis of transverse ligament can lead to instability of atlas on axis. Caution must be used during endotracheal intubation; may see loss of lordosis of the neck and decreased range of motion; C4-C5 and C5-C6 subluxations are possible; may see joint space narrowing on lateral cervical spine films; avoid flexion films until odontoid fracture ruled out if injury is suspected; myelopathy can occur, with gradual onset of upper extremity weakness and paresthesias. |
| Eye problems | Episcleritis rarely occurs. |
| Fistula formation | Cutaneous sinuses form near affected joints, connecting bursa to the skin. |
| Increased infections | More likely to be an effect of rheumatoid arthritis treatment. |
| Hand joint deformities | Ulnar deviation at metacarpophalangeal joints; boutonniere deformity—flexed PIP and hyperextended DIP; swan neck deformity—the reverse of boutonniere, with flexed DIP and hyperextended PIP; thumb hyperextension; increased risk of tendon rupture |
| Other joint deformities | Frozen shoulder may develop; popliteal cysts can arise; carpal and tarsal tunnel syndromes common. |
| Respiratory complications | Lung nodules can coexist with cancers and form cavitary lesions; cricoarytenoid joint inflammation can arise, with hoarseness and laryngeal pain; pleuritis—present in 20 percent at onset of disease; not usually associated with pleuritic pain; interstitial fibrosis—rales may be noted on lung examination. |
| Rheumatoid nodules | Often have necrotic tissue in their centers; found in 20 to 35 percent of patients with rheumatoid arthritis; usually found on extensor surfaces of the limbs or other pressure points; may form nearly anywhere, including on the sclera, vocal cords, sacrum, or vertebral bodies. |
| Vasculitis | Forms include distal arteritis, pericarditis, peripheral neuropathy, cutaneous lesions, arteritis of viscera, and coronary arteritis; increased risk of developing if male sex, high rheumatoid factor titers, treatment with steroids, number of disease-modifying antirheumatic drugs prescribed; associated with increased risk of myocardial infarction. |
Prognosis
Predictors of poor outcomes in the early stages of rheumatoid arthritis include a relatively low functional score early in the disease progression, lower socioeconomic status, lower education level, strong family history of the disease, and early involvement of many joints. Prognosis is worse in patients who have a high ESR or CRP level at disease onset, positive rheumatoid factor, or early radiologic changes.15 Thirty percent of patients with rheumatoid arthritis, usually those with the most severe forms of the disease, will not demonstrate an ACR 20 response (see Duration of Treatment section, above) to any treatment.13 However, patients with milder disease tend to benefit from early treatment. A study of patients with rheumatoid arthritis onset in the 1980s showed no increase in mortality with rheumatoid arthritis in the first eight to 13 years following diagnosis.35 The standardized all-cause mortality ratio for patients with rheumatoid arthritis compared with the general population is 1.6,36 but this may decrease with long-term use of new DMARDs.