
Am Fam Physician. 2005;72(12):2474-2481
The authors thank Michelle Beattie for assistance with the literature review.
Author disclosure: Nothing to disclose.
Because of high incidence, morbidity, and antimicrobial resistance, Staphylococcus aureus infections are a growing concern for family physicians. Strains of S. aureus that are resistant to vancomycin are now recognized. Increasing incidence of unrecognized community-acquired methicillin-resistant S. aureus infections pose a high risk for morbidity and mortality. Although the incidence of complex S. aureus infections is rising, new antimicrobial agents, including daptomycin and linezolid, are available as treatment. S. aureus is a common pathogen in skin, soft-tissue, catheter-related, bone, joint, pulmonary, and central nervous system infections. S. aureus bacteremias are particularly problematic because of the high incidence of associated complicated infections, including infective endocarditis. Adherence to precautions recommended by the Centers for Disease Control and Prevention, especially handwashing, is suboptimal.
Approximately 20 percent of healthy persons are persistent carriers of Staphylococcus aureus, and 60 percent are intermittent carriers. Colonization rates are increased in hemodialysis patients, illicit injection drug users, surgical patients, and patients with insulin-dependent or poorly controlled diabetes.1 The National Nosocomial Infections Surveillance System2 found that 60 percent of hospital-acquired S. aureus isolates in 2003 were methicillin-resistant S. aureus (MRSA). Hospitalized patients with S. aureus infection have five times the risk of in-hospital mortality compared with inpatients without this infection.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Vancomycin (Vancocin) should not be used for known methicillin- susceptible Staphylococcus aureus infections unless there is a betalactam allergy. | C | 10, 11 |
| Physicians should be aware of the regional prevalence of community- acquired MRSA and plan empiric therapy for S. aureus infections accordingly. | C | 8 |
| In patients with bacteremia, non-tunneled central venous catheters and tunneled catheters with tunnel, pocket, or exitsite infection should be removed. | C | 20 |
| All central venous catheters should be removed if a patient has bacteremia for more than 72 hours. | C | 16, 20 |
| Most adult patients with osteomyelitis require four to six weeks of parenteral therapy or prolonged courses (three to six months) of oral antibiotics with high bioavailability. Some children with acute hematogenous osteomyelitis of susceptible organisms respond to a shorter course of parenteral therapy followed by a course of oral therapy. | C | 22, 23 |
| Most infected hardware devices, such as central nervous system shunts, orthopedic fixation devices and prosthetic joints, need to be removed, but limited evidence is available that early stable prosthetic joint infections may respond to long courses of combined quinolone- rifampin (Rifadin) therapy for susceptible organisms. | B | 22, 25 |
| Most abscesses and empyemas require drainage, but limited evidence from case reports is available that some small abscesses of susceptible organisms in clinically stable patients respond to medical therapy without drainage. | C | 28 |
| Hospitalized patients infected or colonized with MRSA should be placed in contact precautions. Use of active surveillance cultures may prevent the spread of MRSA among hospitalized patients. | C | 29–31 |
Community-acquired MRSA infections, which usually cause skin and soft-tissue infections, have become more common.4,5 Occurrence of these infections has increased in athletes, military recruits, children, Pacific Islanders, Alaskan Natives, Native Americans, men who have sex with men, and prison inmates.6
These isolates often are associated with the Panton-Valentine leukocidin and type IV staphylococcal cassette chromosome, which are not typical of hospital-acquired MRSA. Post-influenza pneumonias,7 necrotizing fasciitis, pyomyositis,8 and Waterhouse-Friderichsen syndrome9 caused by community-acquired MRSA also have been observed.
Antimicrobial Therapy
Table 1 lists the costs of antibiotic therapy for S. aureus infections. Antimicrobial therapy should be guided by the susceptibility profile of the organism.6 Beta-lactamase–producing strains of methicillin-susceptible S. aureus (MSSA) preferably are treated with a semi-synthetic penicillin (e.g., intravenous nafcillin, oxacillin [Bactocill], oral dicloxacillin [Dynapen]) in patients not allergic to penicillin. First-generation cephalosporins (e.g., oral cephalexin [Keflex], intravenous cefazolin [Ancef]) are an alternative. Vancomycin (Vancocin) should only be used for the treatment of MSSA in patients allergic to penicillins because of overuse and development of resistant organisms, and because clearance of bacteremia may be slow.10
| Antibiotic | Representative dose | Cost per day (generic)* |
|---|---|---|
| Cephalexin (Keflex) | 500 mg orally every six hours | $14 (2 to 5) |
| Dicloxacillin (Dynapen) | 500 mg orally every six hours | 8 (5 to 7) |
| Trimethoprim/sulfamethoxazole (Bactrim, Septra) | 160 mg/800 mg every 12 hours | 2 (1 to 2) |
| Clindamycin (Cleocin) | 300 mg orally every six hours | 24 (14 to 15) |
| 600 mg intravenously every eight hours | 25 (13) | |
| Linezolid (Zyvox) | 600 mg orally every 12 hours | 130 |
| 600 mg intravenously every 12 hours | 164 | |
| Nafcillin | 2 g intravenously every four hours | 124 |
| Cefazolin (Ancef) | 2 g intravenously every eight hours | 33 (7 to 16) |
| Vancomycin (Vancocin) | 1 g intravenously every 12 hours | 69 (18 to 40) |
| Daptomycin (Cubicin) | 300 mg intravenously once daily | 171 |
Vancomycin is preferred for treatment in severe MRSA infections and is used only intravenously because the oral formulation is not readily absorbed from the gastrointestinal tract. Vancomycin-intermediate susceptible and vancomycin-resistant strains of S. aureus have been reported. Even in patients with vancomycin-susceptible MRSA, there have been reports of treatment failure with vancomycin, which is thought to be because of heterogeneous subpopulations with varying susceptibility to vancomycin,11 or associated with the presence of the regulatory gene agr group II polymorphism.12
Linezolid (Zyvox) has bacteriostatic activity against S. aureus and is approved for treatment of complicated skin and soft-tissue infections and pneumonia in adults and children. It is included in the oxazolidinone class of drugs and has parenteral and oral formulations, with good oral bioavailability. One retrospective analysis13 of a database from a prospective randomized trial suggested enhanced effectiveness of linezolid compared with vancomycin in MRSA nosocomial pneumonia. The rationale may be related to enhanced concentrations of linezolid in lung epithelial lining fluid. The main adverse event associated with linezolid is bone marrow suppression, especially thrombocytopenia, which is increased with dosage and duration of therapy. Coadministration of selective serotonin reuptake inhibitors and adrenergic drugs should be avoided because of central nervous system toxicity.
Daptomycin (Cubicin), from a new class of cyclic lipopeptides, is an antibiotic with activity against MSSA and MRSA. It is rapidly bactericidal against S. aureus in vitro; approved for adults with complicated skin and soft-tissue infections14; highly bound to serum proteins but has poor penetration in lung tissue; and inactivated by surfactant, so it should not be used for pulmonary infections. Creatinine kinase levels should be monitored during therapy because there have been reports of muscle toxicity. Daptomycin only is available for intravenous administration, and the recommended dosage is 4 mg per kg over 30 minutes by intravenous infusion in 0.9 percent sodium chloride once daily for one to two weeks.
Community-acquired MRSA isolates often are susceptible to fluoroquinolones, trimethoprim/sulfamethoxazole (Bactrim, Septra), tetracyclines, and clindamycin (Cleocin). If the infection site is superficial, it may be reasonable to treat it with one of these agents. On the basis of minimal inhibitory concentration testing, community-acquired MRSA may be reported by the laboratory as susceptible to clindamycin and resistant to erythromycin. In these cases, clindamycin may have inducible resistance that could emerge on therapy, so the laboratory can perform a double-disc diffusion test to check for inducible resistance and determine true susceptibility.15
Skin and Soft-Tissue Infections
S. aureus is associated with various skin and soft-tissue infections including folliculitis, impetigo, furuncles, carbuncles, hidradenitis suppurativa, and cellulitis. Management depends on extent of involvement. Wound care and drainage may be all that is necessary in small localized lesions. Localized impetigo may be treated topically with mupirocin (Bactroban). Systemic antibiotics are used for cellulitis or in the presence of systemic symptoms. Short courses (i.e., five to 14 days) are recommended (Table 2). Larger carbuncles or localized abscesses require incision and drainage. Because of the increasing concern of community-acquired MRSA, purulent lesions that require systemic therapy should be cultured so that antimicrobial susceptibility testing can be performed, and initial empiric treatment should consider the local prevalence of community-acquired MRSA.8
| Type of infection | Antibiotic choice | Alternate antibiotic choices | Length of therapy |
|---|---|---|---|
| Simple, uncomplicated skin infections | Five to seven days | ||
| MSSA | Cephalexin (Keflex), dicloxacillin (Dynapen) | Clindamycin (Cleocin) | |
| MRSA | Clindamycin, trimethoprim/sulfamethoxazole (Bactrim, Septra), linezolid (Zyvox) | –– | |
| Complex skin and soft-tissue infections | Two to four weeks (varies) | ||
| MSSA | Nafcillin | Cefazolin (Ancef), clindamycin | |
| MRSA | Vancomycin (Vancocin) | Linezolid, daptomycin (Cubicin) | |
| Bacteremia | Two to four weeks | ||
| MSSA | Nafcillin | Cefazolin, vancomycin | |
| MRSA | Vancomycin | Linezolid, daptomycin | |
| Catheter-related infections | Two weeks, if no infective endocarditis | ||
| MSSA | Nafcillin | Cefazolin, vancomycin | |
| MRSA | Vancomycin | Linezolid, daptomycin | |
| Osteomyelitis | Four to six weeks | ||
| MSSA | Nafcillin, cefazolin | Clindamycin, quinolone plus rifampin (Rifadin) | |
| MRSA | Vancomycin | Linezolid, daptomycin | |
| Pneumonia | 10 to 14 days | ||
| MSSA | Nafcillin | Vancomycin, clindamycin | |
| MRSA | Vancomycin, linezolid | — | |
S. aureus, mediated by toxin production, also can cause toxic shock syndrome and staphylococcal scalded skin syndrome. Toxic shock syndrome manifests as fever, hypotension, a macular rash that later desquamates, and multiple organ dysfunction. Management includes removal of the focus of S. aureus (e.g., abscess drainage or tampon removal) and use of a betalactamase–resistant antistaphylococcal antibiotic in combination with clindamycin, which has the potential of reducing toxin production. Management of staphylococcal scalded skin syndrome often requires intravenous antibiotics and potentially drainage of lesions, which are the basis of the infection with the toxin-producing strains.
Bacteremia
S. aureus bacteremia may lead to several complications including infective endocarditis, sepsis, or metastatic foci of infection. About 12 percent of patients with S. aureus bacteremia have infective endocarditis.16 Transesophageal echocardiography is superior to transthoracic echocardiography in diagnosis of perivalvular abscess, prosthetic valve involvement, and recognizing smaller vegetations. Transthoracic echocardiography helps secure the diagnosis of infective endocarditis and predict serious intracardiac complications.17 A cost-effectiveness study18 suggested that in clinically uncomplicated catheter-associated S. aureus bacteremia, the use of transesophageal echocardiography was cost-effective compared with two or four weeks of empiric antimicrobial therapy, although this issue remains controversial. Consultation with an infectious diseases sub-specialist may be beneficial.19 An algorithm for the management of S. aureus bacteremia is provided in Figure 1.
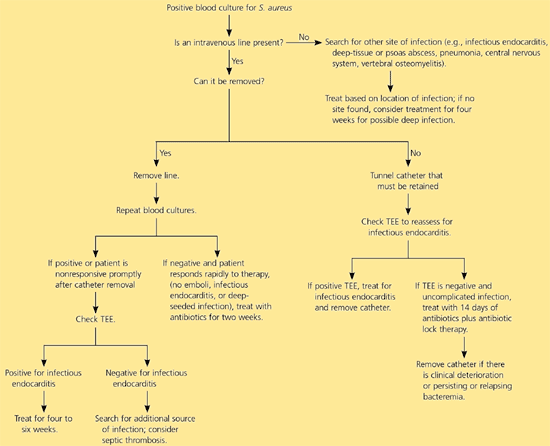
Catheter-Related Infections
Guidelines from the Infectious Diseases Society of America20 recommend removal of non-tunneled central venous catheters associated with S. aureus bacteremia. A tunneled (i.e., Hickman catheter) or implantable device should be removed if there is purulence or erythema at the exit site or along the tunnel, evidence of pocket infection, or if it is associated with a complicated deep-seated infection. Transesophageal echocardiography is recommended in the evaluation of catheter-related bloodstream infections. In the absence of endocarditis, septic phlebitis, or deep-seated infection, 14 days of systemic antimicrobial therapy is recommended.20 Attempting to salvage the catheter in patients with uncomplicated infections should include antibiotic lock therapy (i.e., filling the catheter lumen with high concentrations of antibiotics and leaving them there for hours to days) with two weeks of parenteral antimicrobial therapy.20 The catheter should be removed if there is persistent bacteremia for 72 or more hours of therapy, clinical deterioration, or relapse of bacteremia. A beta-lactam (i.e., nafcillin) is the drug of choice for patients with MSSA not allergic to penicillin; vancomycin is preferred for MRSA catheter-associated infections.20
Osteomyelitis
S. aureus is the most commonly isolated micro-organism in osteomyelitis, and more than one third of these isolates may be MRSA.21 Hematogenous spread of S. aureus can lead to vertebral osteomyelitis and potentially epidural abscess formation. Treatment for S. aureus osteomyelitis should include at least four to six weeks of antimicrobial therapy.22 Patients with vertebral osteomyelitis, especially in the presence of neurologic symptoms, should be evaluated with magnetic resonance imaging for epidural abscess formation. In children, hematogenous spread often causes long bone osteomyelitis. Short courses (i.e., two weeks) of intravenous antibiotics followed by another two to four weeks of oral antibiotics may be used in children who respond promptly to initial antibiotics and have no complications.23 In addition to prolonged antimicrobial therapy, surgical therapy usually is required in osteomyelitis secondary to a contiguous focus of infection, usually observed after orthopedic surgery or trauma. Infected hardware generally requires removal, which may be delayed with use of oral antimicrobials until stability is ensured if there is bone nonunion.24
Joint Infections
S. aureus is a major pathogen in joint infections. Although there is limited evidence regarding treatment, it usually is managed using drainage combined with a four-week course of antimicrobials. During the last two weeks, antimicrobials sometimes are given orally to patients without bacteremia. Prosthetic joint infections are difficult to eradicate with the foreign material in place and usually require removal of the prosthesis followed by antibiotics for four to six weeks to treat the infection. Limited data indicate that early-onset infected joint prostheses may be treated with early debridement and prolonged courses of a quinolone plus rifampin (Rifadin) without prosthesis removal.25
Pulmonary Infections
S. aureus pneumonia may be caused by hematogenous spread or aspiration and is a common pathogen in nosocomial pneumonias. S. aureus community-acquired pneumonia occurs more commonly after influenza infection, and community-acquired MRSA associated with the Panton-Valentine leukocidin has been described.7 Results of chest radiographs can vary in presentation from localized consolidation to abscess to multilobe diffuse infiltrates. Empyema is caused by extension of local pneumonia. Therapy includes chest-tube drainage, and thorascopic or open drainage occasionally is required.26
Central Nervous System Infections
S. aureus is estimated to cause approximately 2 percent of all bacterial meningitis cases from a hematogenous or postoperative source. Eighty-four percent of patients with postoperative S. aureus meningitis had a catheter in place, typically a shunt or epidural catheter.27 Such devices need to be removed and replaced after the infection has cleared.
S. aureus causes about 10 to 15 percent of brain abscesses and 60 to 90 percent of epidural abscesses and septic venous thromboses. Surgical or radiographic drainage usually is required, but some small abscesses in patients without neurologic deficits have responded to medical therapy.28
Prevention
Contact precautions recommended by the Centers for Disease Control and Prevention for hospitalized patients with MRSA include use of a private room, wearing gloves on entering the room, wearing a gown if contact with the patient or items in the room is anticipated, and handwashing on removal of the gloves.29 Despite these guidelines, nosocomial MRSA has been increasing in frequency.2 It is not clear that these guidelines are effective in controlling MRSA,30,31 and well-designed randomized controlled studies have not been performed. Adherence to the guidelines has been suboptimal, and hand-washing in particular is inadequate. About 5 percent of hospitalized patients are colonized with MRSA. Advocates have suggested that more active surveillance using preemptive isolation and screening of patients, with stricter adherence to contact precautions and handwashing, may be more successful.29
Topical mupirocin is effective in reducing nasal colonization of S. aureus,32 but the use of topical mupirocin to reduce the risk of surgical or nonsurgical infections caused by S. aureus has not been reliably successful.33,34 It also is not clear that topical mupirocin reduces MRSA colonization.35 Attempts at combining topical mupirocin with antibacterial baths (e.g., in chlorhexidine or systemic agents) deserve further study.