Am Fam Physician. 2005;72(12):2545-2548
In a recent survey by the American Academy of Family Physicians (AAFP), active members overwhelmingly identified American Family Physician as their single most used source of clinical immunization information (81 percent; AAFP Immunization Survey, unpublished data, 2005). The 2006 Recommended Adult Immunization Schedule—as approved by the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, the AAFP, and the American College of Obstetricians and Gynecologists—provides a concise tool to help family physicians identify who to vaccinate, when to vaccinate, and which vaccine to use.
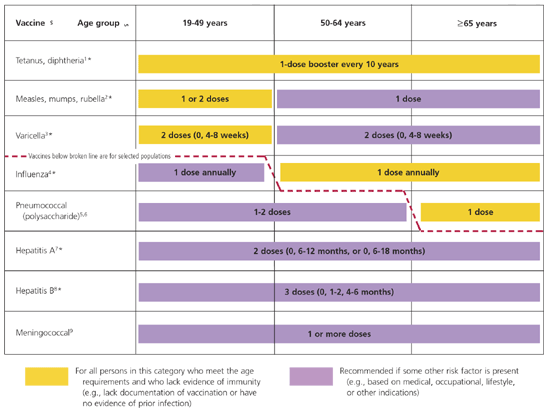
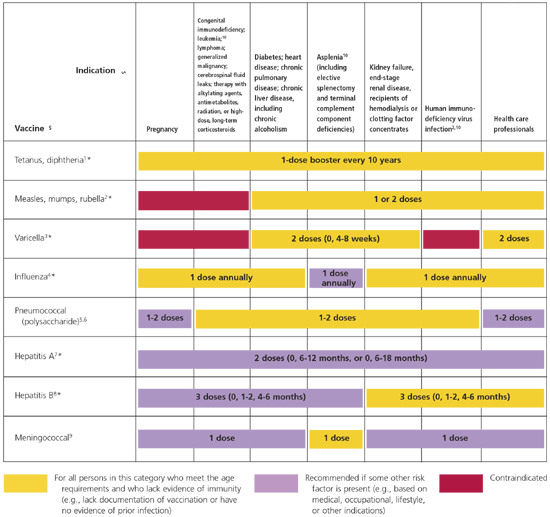
The 2006 schedule has been simplified and is depicted in two charts (Figures 1 and 2). The first chart provides age-based recommendations for two categories of patients: (1) those who meet age requirements and lack evidence of immunity, and (2) those in whom some other risk factor is present. The second chart provides guidance for seven risk groups comprised of pregnant women; immunocompromised persons; health care professionals; and persons with chronic medical conditions, asplenia, renal failure, and human immunodeficiency virus infection.
The benefits of immunization often extend well beyond individual patients. For example, a recent systematic review1 of pneumococcal vaccination in adults 55 years and older demonstrated 53 percent effectiveness in reducing rates of invasive pneumococcal disease. Beyond this protection, immunization of children with 7-valent pneumococcal conjugate vaccine (PNC7; Prevnar) has been associated with a 55 percent decrease in invasive pneumococcal disease in adults 50 years and older between 1998 and 2003, thus demonstrating the role of herd immunity.2
Recent examples of a measles outbreak in Indiana3 and of polio infections in Minnesota,4 both occurring in undervaccinated communities, serve as testimony to the ongoing role of vaccination when highly contagious diseases are only an airplane ride away. One of the patients with measles was hospitalized with pneumonia and required six days of ventilator support.
Effective, evidence-based methods to improve immunization rates in ambulatory practice include reminder systems, assessment and feedback to vaccine providers, and standing orders.5 Despite their known effectiveness, these tools are underused by family physicians and have estimated use rates of 46, 31, and 67 percent, respectively (AAFP Immunization Survey, unpublished data, 2004). Such interventions can be implemented easily by primary care physicians.
Family physicians are encouraged to review the 2006 adult immunization schedule and the accompanying footnotes. To maximize the patient-centered benefits, however, this schedule should be coupled with clinical interventions that have been shown to effectively increase immunization rates.