
Am Fam Physician. 2006;73(4):669-676
Patient information: See related handout on chronic obstructive pulmonary disease, written by the authors of this article.
Author disclosure: Nothing to disclose.
Chronic obstructive pulmonary disease is characterized by the gradual progression of irreversible airflow obstruction and increased inflammation in the airways and lung parenchyma that is generally distinguishable from the inflammation caused by asthma. Most chronic obstructive pulmonary disease is associated with smoking, but occupational exposure to irritants and air pollution also are important risk factors. Patients with chronic obstructive pulmonary disease typically present with coughing, sputum production, and dyspnea on exertion. However, none of these findings alone is diagnostic. The Global Initiative for Chronic Obstructive Lung Disease diagnostic criterion for chronic obstructive pulmonary disease is a forced expiratory volume in one second/forced vital capacity ratio of less than 70 percent of the predicted value. Severity is further stratified based on forced expiratory volume in one second and symptoms. Chest radiography may rule out alternative diagnoses and comorbid conditions. Selected patients should be tested for α1-antitrypsin deficiency. Arterial blood gas testing is recommended for patients presenting with signs of severe disease, right-sided heart failure, or significant hypoxemia. Chronic obstructive pulmonary disease also is a systemic disorder with weight loss and dysfunction of respiratory and skeletal muscles.
The global burden of chronic obstructive pulmonary disease (COPD) is increasing; the disease is projected to be the third leading cause of death and fifth leading cause of overall disability worldwide by 2020.1 Men and women seem to be at an equal risk, and the death rate attributable to COPD is increasing significantly in both sexes.1,2 The economic consequences of COPD are substantial. In 2002, the estimated total societal cost of COPD in the United States was $32 billion.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Spirometry should be used as the first-line diagnostic tool when evaluating patients for COPD. A FEV1/FVC ratio of less than 70 percent of the predicted value suggests COPD. | C | 5,6 |
| Patients with suspected COPD should receive chest radiography to exclude other diagnoses and comorbidities. | C | 6,31 |
| α1-Antitrypsin deficiency testing should be performed in select patients (e.g., COPD in never-smokers, idiopathic cirrhosis, family history of α1-antitrypsin deficiency, predominantly lower lung emphysema, “premature” COPD, and refractory asthma at a young age). | C | 15 |
| ABG testing should be performed if the patient presents with right-sided heart failure or has more severe COPD. | C | 5 |
Definition
COPD is a heterogeneous disorder that encompasses traditional clinical entities such as emphysema and chronic bronchitis.3,4 The Global Initiative for Chronic Obstructive Lung Disease (GOLD),5 a collaborative effort from the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the World Health Organization, defines COPD as a usually progressive disease with airflow limitation that is not fully reversible and that is associated with an abnormal inflammatory response of the lungs to noxious particles or gases.
Patients with COPD present with a variety of clinical findings, including elements of chronic bronchitis and emphysema.6–8 Although COPD and asthma are both associated with airflow obstruction and inflammation of the lung and airways, asthma-related airflow obstruction is more reversible and the disease course is more variable than with COPD.6,8,9
Risk Factors
Exposure to tobacco smoke is the most significant risk factor for COPD, with 80 to 90 percent of all cases attributable to smoking.6 Evidence linking tobacco smoke exposure and COPD predominantly comes from population-based studies that have consistently shown that smoking is associated with diminished lung function, more frequent respiratory symptoms, and increased COPD-related deaths.5,10–13 Pipe and cigar smoking are associated with increased COPD risk, but at a lesser rate than with cigarette smoking.5,6 Although cigarette smoking is a significant risk factor for COPD, only about 20 percent of cigarette smokers develop clinically significant COPD.6,8,13
The second most significant documented risk factor for COPD is α1-antitrypsin deficiency. Although α1-antitrypsin deficiency increases the risks associated with smoking, COPD can develop in never-smokers with α1-antitrypsin deficiency. One percent of COPD cases are attributable to severe α1-antitrypsin deficiency.5,6,14,15
| Occupation | Irritant |
|---|---|
| Agricultural worker | Endotoxin |
| Coal miner | Coal dust |
| Concrete worker | Mineral dust |
| Construction worker | Dust |
| Gold miner | Silica |
| Hard rock miner | Mineral dust |
| Rubber worker | Industrial chemicals |
Natural History
Patients with COPD may present with loss of lung function beyond normal age-related decreases. Clinical disease develops fairly late in the disease course, after lung function drops below threshold values.
After 25 years of age, a nonsmoking adult’s forced expiratory volume in one second (FEV1) decreases by an average of 20 to 40 mL per year. In some smokers, FEV1 decreases by two to five times this amount, making them particularly susceptible to COPD.6,8,12,13,21,22 Smoking cessation may cause slight initial improvement in FEV1 (approximately 50 mL in the first year).21 More importantly, smoking cessation can give a former smoker the same average ongoing loss of lung function as a never-smoker.13,21 Figure 113,21 illustrates the progressive loss of lung function in a variety of settings.
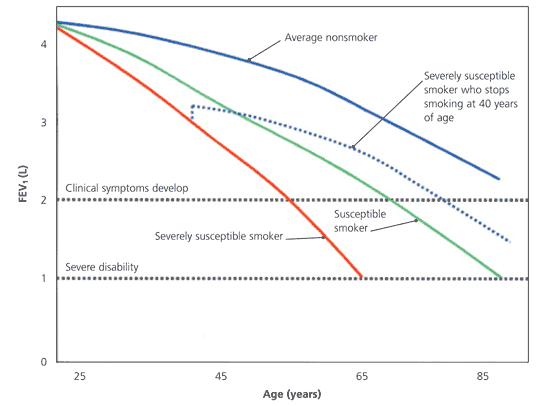
Patients with COPD usually have a smoking history of at least 20 pack-years. Wheezing and dyspnea on exertion generally occur when the FEV1 is less than 50 percent of the predicted value, and significant physical disability usually occurs when the FEV1 is less than 35 to 40 percent of the predicted value.4,22,23 Patients who started smoking in their 20s and have already sustained appreciable FEV1 loss by their 40s are likely to develop significant COPD if they continue to smoke. Those in their mid-40s who have normal FEV1 values, however, probably will not develop symptomatic disease.13
Pathophysiology
COPD involves a chronic inflammatory process, primarily in the peripheral airways and the lung parenchyma. Airway irritation causes mucous gland enlargement, hypersecretion, ciliary dysfunction, and squamous metaplasia early in the disease course. An ongoing cycle of inflammation and repair ultimately narrows the airway lumen and obstructs airflow. Patients with emphysema experience destruction of the structural constituents of the alveolar walls, causing permanent enlargement of the air spaces distal to the terminal bronchioles. In early COPD, emphysematous changes are most prominent in the upper lung fields. Loss of the structural supports that keep the airways open allows the bronchioles to collapse during expiration; the resulting nonfunctioning alveolar units reduce the amount of lung area available for gas exchange.4–6,24
Diagnosis
Common differential diagnosis of COPD includes asthma, heart failure, bronchiectasis, bronchiolitis obliterans, cystic fibrosis, and tuberculosis (Table 2). Clinical history; physical examination; and diagnostic testing, such as lung function measurements, can help diagnose COPD. Chest radiography may rule out alternative diagnoses and comorbid conditions.
| Diagnosis | Characteristics | Clinical presentation | Pulmonary function test findings | Chest radiography findings | Other recommended testing |
|---|---|---|---|---|---|
| COPD | Midlife to late-life onset; steadily progressive with exacerbations; associated with smoking history | Chronic productive cough, dyspnea, and wheezing | Predominantly fixed airflow obstruction, decreased DLCO | Hyperinflation, increased basilar markings, bronchial thickening | α1-Antitrypsin testing, ABG testing, and chest CT in selected patients |
| Asthma | Usually early-life onset; episodic; associated with other allergic disorders and family history | Episodic wheezing, cough, and dyspnea | Predominantly reversible airflow obstruction, normal DLCO | Normal between episodes | Allergy testing, peak- flow monitoring |
| Bronchiectasis | Usually midlife onset; progressive with exacerbations | Productive cough with thick, purulent sputum; dypsnea; and wheezing | Obstructive airflow limitation, both fixed and reversible | Focal pneumonia, atelectasis; dilated, thickened airways (ring shadow) | Bacterial, microbacterial, and fungal sputum culture, chest CT |
| Bronchiolitis obliterans | Onset at any age; may be associated with history of flu-like illness, collagen vascular disease, or toxic exposure | Often subacute presentation with dyspnea, cough, and fever | Decreased vital capacity, decreased DLCO, usually no obstructive component | Multifocal, bilateral alveolar infiltrates | ESR, high-resolution CT, lung biopsy |
| Congestive heart failure | Midlife to late-life onset; associated with risk factors such as hypertension and coronary artery disease | Fatigue, exertional and paroxysmal nocturnal dyspnea, and peripheral edema | Decreased DLCO, predominantly used to exclude other diagnoses | Increased heart size, pulmonary vascular congestion, pleural effusions | Echocardiography, BNP measurement, electrocardiography; cardiac catheterization in selected patients |
| Tuberculosis | Onset at any age; associated with history of exposure | Productive cough, hemoptysis, fever, and weight loss | Not used for diagnosis | Infiltrate, nodular lesions, hilar adenopathy | Sputum AFB culture |
| Cystic fibrosis | Usually early-life onset; progressive with exacerbations; associated with pancreatic disease, failure to thrive, intestinal obstruction, cirrhosis, and steatorrhea | Predictive cough with purulent sputum, dyspnea, and wheezing | Predominantly fixed airflow obstruction | Bronchiectasis frequent in upper lobes | Sweat chloride test (diagnostic), Bacterial sputum culture |
CLINICAL HISTORY
Patients with COPD typically present with cough, sputum production, and dyspnea on exertion. A COPD diagnosis is likely if a patient with a significant history of tobacco smoke exposure has these symptoms. Patients with COPD also may experience orthopnea soon after reclining, unlike patients with heart failure, in whom orthopnea typically occurs hours after reclining, when fluid mobilizes from the lower extremities. COPD-associated hemoptysis often is caused by airway mucosal erosion from coughing, but a coexisting cancer or underlying infection (e.g., tuberculosis) should be considered. Severe nocturnal hypoxia or hypercapnia should be considered if a patient with COPD presents with a persistent morning headache.
PHYSICAL EXAMINATION
Patients with COPD often present with diminished breath sounds, prolonged expiratory time, and expiratory wheezing that initially may occur only on forced expiration. Additional findings on physical examination include hyperinflation of the lungs with an increased anteroposterior chest diameter (“barrel chest”); use of accessory muscles of respiration; and distant heart sounds, sometimes best heard in the epigastrium. Patients with more advanced disease may have pursed lip breathing or postures that relieve dyspnea (e.g., leaning forward against outstretched palms). The presence of significant edema may indicate right-sided heart failure and cor pulmonale in patients with pulmonary hypertension from severe long-standing COPD.
The literature on the effectiveness of specific components of the patient history and physical examination as predictors of obstructive airway disease is of limited value. A history of COPD and a significant smoking history generally are strong predictors of airway obstruction, and the clinical likelihood of COPD is greater when multiple symptoms or signs suggesting COPD are present.24–28 Table 324 lists the predictive values of common physical symptoms and signs for detecting airflow obstruction.
| Signs/symptoms | Sensitivity (%) | Specificity (%) | +LR | –LR |
|---|---|---|---|---|
| No smoking history versus smoking history | 92 | 49 | 1.8 | 0.16 |
| ≥ 70 pack-years versus < 70 pack-years | 40 | 95 | 8.0 | 0.63 |
| Wheezing history | 51 | 84 | 3.8 | 0.66 |
| Dyspnea | 82 | 33 | 1.2 | 0.55 |
| Coughing | 51 | 71 | 1.8 | 0.69 |
| Wheezing on examination | 15 | 99.6 | 36.0 | 0.85 |
| Decreased breath sounds on examination | 37 | 90 | 3.7 | 0.70 |
DIAGNOSTIC TESTING
The best diagnostic test for evaluating patients with suspected COPD is lung function measured with spirometry. The GOLD guidelines5 characterize the severity of COPD according to clinical and spirometric measures(Table 45). Key spirometric measures may be obtained with a portable office spirometer and should include forced vital capacity (FVC) and FEV1.Patients with COPD typically present with obstructive airflow. According to the GOLD criteria, a FEV1/FVC ratio of less than 70 percent in a patient with a postbronchodilator FEV1 of less than 80 percent of the predicted value is diagnostic for COPD.5 Severity is further stratified based on symptoms and FEV1 values. A patient with severe disease has a FEV1 of less than 50 percent of the predicted value; values below 30 percent of the predicted value represent very severe disease.5,6,29 Although some experts have proposed periodic FEV1 testing for high-risk patients older than 45 years to facilitate risk factor reduction counseling, no evidence exists to support this recommendation.22
| Stage | Description | Findings* | |
|---|---|---|---|
| 0 | At risk | Risk factors and chronic symptoms but normal spirometry | |
| I | Mild | FEV1/FVC ratio less than 70 percent | |
| FEV1 at least 80 percent of predicted value | |||
| May have symptoms | |||
| II | Moderate | FEV1/FVC ratio less than 70 percent | |
| FEV1 50 percent to less than 80 percent of predicted value | |||
| May have chronic symptoms | |||
| III | Severe | FEV1/FVC ratio less than 70 percent FEV1 30 percent to less than 50 percent of predicted value | |
| May have chronic symptoms | |||
| IV | Very severe | FEV1/FVC ratio less than 70 percent | |
| FEV1 less than 30 percent of predicted value | |||
| or | |||
| FEV1 less than 50 percent of predicted value plus severe chronic symptoms | |||
Beyond office spirometry, complete pulmonary function testing may show increased total lung capacity, functional residual capacity, and residual volume. A substantial loss of lung surface area available for effective oxygen exchange causes diminished carbon monoxide diffusion in the lung (DLCO) in patients with emphysema. This finding may help distinguish COPD from asthma, because patients with asthma typically have normal DLCO values.5,6,30
Chest radiography usually is abnormal in patients with severe COPD but may not show changes in up to one half of patients with moderate disease.6,31 Chest radiography in patients with COPD may show hyperinflation of the lungs, flattening of the domes of the diaphragm, and tapering of the pulmonary vessels as they move to the periphery of the lung fields. Increased basilar markings (“dirty lungs”) sometimes are visible on chest radiographs in patients with chronic bronchitis, and isolated bullae may be seen in patients with emphysema. A computed tomography (CT) scan of the chest should not be used routinely to diagnose COPD, but it may show findings that are highly correlated with the degree of COPD changes found on pathologic examination.31
Testing for α1-antitrypsin deficiency is appropriate in selected patients; testing involves measuring circulating α1-antitrypsin levels followed by phenotype testing if levels are abnormal. Patients with severe α1-antitrypsin deficiency usually are of European descent and develop clinical evidence of COPD approximately 10 years earlier than patients who are not α1-antitrypsin deficient.6Lung changes associated with severe α1-antitrypsin deficiency usually include lower lung field predilection. α1-Antitrypsin deficiency also may cause otherwise unexplained cirrhosis of the liver.6,14,15,19 Clinical circumstances in which testing for α1-antitrypsin deficiency should be considered include COPD in never-smokers, idiopathic cirrhosis, family history of α1-antitrypsin deficiency, predominantly lower lung emphysema, “premature” COPD, and refractory asthma at a young age.6,15
Arterial blood gas measurement is recommended to rule out significant hypoxemia (partial pressure of oxygen less than 60 mm Hg) or hypercapnia in patients with more severe disease. This is based on FEV1 (less than 40 percent of predicted value), signs of right-sided heart failure, and signs of hypoxemia.5
Clinical Subtypes
Patients with COPD also may be grouped into clinical subtypes based on clinical findings. The most common clinical subtypes are chronic bronchitis and emphysema.
CHRONIC BRONCHITIS WITH AIRFLOW OBSTRUCTION
Pure chronic bronchitis is clinically defined as an otherwise unexplained chronic, productive cough for at least three months in each of two successive years. If fixed airway obstruction is present in a patient with chronic bronchitis, the patient also has COPD. Eighty-five percent of patients with COPD have chronic bronchitis.5 These patients experience prominent cough and sputum production and frequently develop hypoxia and hypercarbia at rest and significant oxygen desaturation during sleep. Patients with chronic bronchitis and COPD often develop pulmonary hypertension, causing right-sided heart failure and cor pulmonale with marked peripheral edema.5–8
EMPHYSEMA
Dyspnea is the most prominent symptom associated with emphysema-related COPD. Hypoxia is less marked than in the bronchitic form of COPD, and hypercarbia is relatively uncommon until very late in the disease course.
Tidal breathing occurs at high lung volumes in patients with emphysema-related COPD to compensate for high pulmonary residual volumes and to open collapsed airways. This produces hyperinflation of the lungs and thorax, greatly increasing the total work required to breathe. Patients with emphysema-related COPD are less likely to have right-sided heart failure and cor pulmonale than patients with chronic bronchitis and COPD.5–7,30,32
COPD as a Systemic Disease
COPD increasingly is considered a systemic disorder with important nonpulmonary components. Weight loss in patients with COPD may be related to increased circulating levels of inflammatory mediators (e.g., tumor necrosis factor alpha, inflammatory cytokines). Pulmonary cachexia associated with severe COPD also causes profound weight loss, which is a predictor of increased mortality risk independent of lung function.5,6,8,25,33,34
Respiratory and skeletal muscle abnormalities accompany COPD. The respiratory muscles of patients with COPD are chronically overworked and fatigued, whereas the extremity muscles tend to be underworked and atrophied.