
Am Fam Physician. 2006;73(7):1198-1204
Patient information: See related handout on abdominal aortic aneurysms, written by Jill Giordano, Medical Editing Clerk at Georgetown University Medical Center.
Author disclosure: Nothing to disclose.
Most abdominal aortic aneurysms (AAAs) are asymptomatic, not detectable on physical examination, and silent until discovered during radiologic testing for other reasons. Tobacco use, hypertension, a family history of AAA, and male sex are clinical risk factors for the development of an aneurysm. Ultrasound, the preferred method of screening, is cost-effective in high-risk patients. Repair is indicated when the aneurysm becomes greater than 5.5 cm in diameter or grows more than 0.6 to 0.8 cm per year. Asymptomatic patients with an AAA should be medically optimized before repair, including institution of beta blockade. Symptomatic aneurysms present with back, abdominal, buttock, groin, testicular, or leg pain and require urgent surgical attention. Rupture of an AAA involves complete loss of aortic wall integrity and is a surgical emergency requiring immediate repair. The mortality rate approaches 90 percent if rupture occurs outside the hospital. Although open surgical repair has been performed safely, an endovascular approach is used in select patients if the aortic and iliac anatomy are amenable. Two large randomized controlled trials did not find any improvement in mortality rate or morbidity with this approach compared with conventional open surgical repair.
Abdominal aortic aneurysm (AAA) is a relatively common and often fatal condition that primarily affects older patients. AAAs and 15,000 deaths yearly and in 2000 were the 10th leading cause of death in white men 65 to 74 years of age in the United States.1 With an aging population, the incidence and prevalence of AAA is certain to rise. Most AAAs are asymptomatic, and physical examination lacks sensitivity for detecting an aneurysm.2 It is important that family physicians understand which patients are at risk for the development of AAA and the appropriate evaluation once a patient has been diagnosed with an aneurysm.
Definition and Etiology
An aneurysm is a permanent focal dilatation of an artery to 1.5 times its normal diameter. The normal infrarenal aortic diameters in patients older than 50 years are 1.5 cm in women and 1.7 cm in men. By convention, an infrarenal aorta 3 cm in diameter or larger is considered aneurysmal.3
The primary event in the development of an AAA involves proteolytic degradation of the extracellular matrix proteins elastin and collagen. Various proteolytic enzymes, including matrix metalloproteinases, are critical during the degradation and remodeling of the aortic wall.4 Oxidative stress also plays an important role, and there is an autoimmune component to the development of AAA, with extensive lymphocytic and monocytic infiltration with deposition of immunoglobulin G in the aortic wall.4 Cigarette smoking elicits an increased inflammatory response within the aortic wall.5 An infectious etiology with Chlamydia pneumoniae has been proposed but not proven.4 Increased biomechanical wall stress also contributes to the formation and rupture of aneurysms with increased wall tension and disordered flow in the infrarenal aorta.4 Finally, 12 to 19 percent of first-degree relatives, predominantly men, of a patient with an AAA will develop an aneurysm.6
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Ultrasound should be used to screen for the presence of AAA in men 65 to 75 years of age who have ever smoked, and it can be considered for patients with a strong family history of AAA. | B | 7,8 |
| Beta blockade, with a goal resting heart rate of 60 beats per minute, should be instituted before AAA repair in all patients unless contraindicated. | A | 17,26–29 |
| Repair of an AAA should be considered when the aneurysm reaches 5.5 cm in maximal diameter in men. | A | 18,19,21 |
| Repair of an AAA also should be considered when the aneurysm expands by more than 0.6 to 0.8 cm per year. | C | 21,22 |
Screening
Ultrasound is the standard imaging tool; if performed by trained personnel, it has a sensitivity and specificity approaching 100 and 96 percent, respectively, for the detection of infrarenal AAA7 (Figure 1). The U.S. Preventive Services Task Force has released a statement summarizing recommendations for screening for AAA.8 It stated that screening benefits patients who have a relatively high risk for dying from an aneurysm; major risk factors are age 65 years or older, male sex, and smoking at least 100 cigarettes in a lifetime. The guideline recommends one-time screening with ultrasound for AAA in men 65 to 75 years of age who have ever smoked. No recommendation was made for or against screening in men 65 to 75 years of age who have never smoked, and it recommended against screening women. Men with a strong family history of AAA should be counseled about the risks and benefits of screening as they approach 65 years of age.
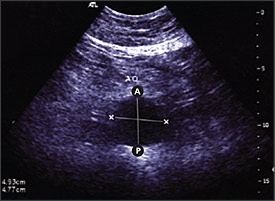
Clinical Evaluation
ASYMPTOMATIC PATIENTS
Most patients with AAA are asymptomatic. Typically, aneurysms are noted on studies performed for other reasons, as opposed to during physical examination. In these patients, it is important to confirm that there is no evidence of significant back, abdominal, or groin pain.
The medical, social, and family history are important in determining if risk factors for development, expansion, and rupture of an aneurysm are present (Table 16,9–14). Previous abdominal operations can make open AAA repair technically difficult and may necessitate a retroperitoneal approach instead of a transabdominal approach. A history of endovascular AAA repair also is important because AAA rupture following endograft repair has been reported.15 Aneurysms proximal and distal to a previous graft (e.g., synchronous AAA) also may occur and present as a pulsatile abdominal mass.
| AAA event | Risk factors |
|---|---|
| Development | Family history (predominantly in men)6; hypercholesterolemia9; hypertension9; male sex9; tobacco use9 |
| Expansion | Advanced age (older than 70 years)10; cardiac or renal transplant11; previous stroke10; severe cardiac disease10; tobacco use10 |
| Rupture | Cardiac or renal transplant11; decreased forced expiratory volume in one second12; female sex (two- to fourfold increase in risk of rupture)12; higher mean blood pressure12; larger initial AAA diameter13; current tobacco use (length of time smoking is more significant than amount smoked)14 |
Abdominal examination in a patient with a suspected AAA should include deep manipulation to elicit pain on aortic palpation. The abdominal aorta may be palpated as part of a normal physical examination without being frankly aneurysmal. However, AAAs in the 3- to 3.9-cm range are palpable 29 percent of the time, whereas those greater than 5 cm are palpable 76 percent of the time.2
The common iliac arteries also may be aneurysmal and palpable in the lower abdominal quadrants. Patients should be examined for the presence of femoral and popliteal pulses and possible aneurysmal dilatation. The presence of a prominent popliteal or femoral artery pulse warrants an abdominal ultrasound to rule out an AAA and a lower extremity arterial ultrasound to rule out peripheral artery aneurysm. There is a 62 percent chance that an AAA is present with a popliteal aneurysm and an 85 percent chance it is present with a femoral artery aneurysm; 14 percent of patients with a known AAA will have a femoral or popliteal artery aneurysm.16
Patients who are diagnosed with an AAA, deny pain, and are clinically stable should be triaged based on the size of the aneurysm (Figure 217). Two large prospective studies18,19 determined independently that surveillance in compliant male patients with an aneurysm 4 to 5.5 cm wide is safe; surgery on AAAs smaller than 5.5 cm did not confer any survival advantage.
Patients with aneurysms greater than or equal to 5.5 cm should be considered for elective AAA repair. Because most clinically diagnosed AAAs are repaired, their long-term natural history is difficult to predict. The one-year incidence of rupture is 9 percent for aneurysms 5.5 to 6.0 cm in diameter, 10 percent for 6.0 to 6.9 cm, and 33 percent for AAAs of 7.0 cm or more.8 Patients with an aneurysm less than 5.5 cm in diameter should have follow-up serial ultrasounds (Table 27). These recommendations serve only as guidelines; each patient should be evaluated for the presence of risk factors for accelerated AAA growth and rupture (Table 16,9–14) and for surgical risk and overall health. A validated clinical decision rule has been published that can help physicians determine a patient’s perioperative mortality risk based on individual risk factors and the overall complication rate.20
| Diameter of aneurysm | Interval for follow-up |
|---|---|
| Less than 3 cm | No further testing |
| 3 to 4 cm | Every 12 months |
| 4 to 4.5 cm | Every six months |
| Greater than 4.5 cm | Consider referral to vascular subspecialist. |
Traditionally, if the AAA expands by more than 0.6 to 0.8 cm per year, the patient should be offered repair.21,22 A recent study23 examining expansion as a criterion for repair failed to find a benefit in a small group of patients; further studies are needed. All patients with AAAs should be educated on the signs of symptomatic and ruptured aneurysms. If they experience new or unusual pain in the back, groin, testicles, legs, or buttocks, emergent medical attention should be sought.
SYMPTOMATIC PATIENTS
In patients presenting with back, abdominal, or groin pain in the presence of a pulsatile abdominal mass, the aorta needs to be evaluated urgently. If the patient is clinically stable, an urgent computed tomography (CT) scan should be obtained (Figure 3) to determine the presence and size of the aorta, as well as to rule out a ruptured AAA. Outcomes for repair of symptomatic AAAs are significantly worse than for asymptomatic aneurysms. One study24 noted a mortality rate of 26 percent in patients with symptomatic AAAs compared with 5 percent for asymptomatic AAAs and 35 percent for ruptured AAAs. Postoperative morbidity of patients undergoing symptomatic AAA repair is similar to those undergoing ruptured AAA repair.24 This underscores the importance of intervention at a point where the risk of rupture is greater than the risk of surgery, but before symptoms occur.
PATIENTS WITH RUPTURED AAA
The classic presentation of a ruptured AAA includes the triad of hypotension, abdominal or back pain, and a pulsatile abdominal mass. In a study25 of 116 patients with ruptured AAAs, 45 percent were hypotensive, 72 percent had pain, and 83 percent had a pulsatile abdominal mass. Patients with ruptured AAAs need immediate intervention to prevent death. Despite advances in perioperative care leading to significant decreases in mortality following AAA repair in asymptomatic patients, postoperative mortality following ruptured AAA repair is still more than 40 percent in patients who survive the operation.13 In general, high-volume hospitals (i.e., performing more than 30 AAA repairs per year) have lower mortality rates, as well as fewer postoperative complications, compared with low-volume hospitals following elective AAA repair.13 If given the option, it may be beneficial to refer patients with symptomatic or ruptured AAAs to high-volume centers when availability and time allow.
Medical Optimization
When evaluating asymptomatic patients before AAA repair, it is important to optimize their comorbidities, particularly cardiac, pulmonary, and renal functions. Patients with coronary artery disease should undergo beta blockade.26 In a study27 of high-risk patients undergoing vascular surgery, bisoprolol (Zebeta) was administered at a dosage of 5 mg once daily at least one week preoperatively. The dosage was increased to a maximum of 10 mg once daily if resting heart rate was still greater than 60 beats per minute approximately one week after institution of therapy. Bisoprolol was withheld if the heart rate was less than 50 beats per minute or the systolic blood pressure fell to less than 100 mm Hg. Death from cardiac causes or nonfatal myocardial infarction occurred in 3.4 percent of the bisoprolol group and 34 percent of the standard-care group (P > .001). A recent meta-analysis28 has confirmed this benefit in high-risk patients but not in low-risk patients.
The American College of Cardiology/American Heart Association Task Force on Practice Guidelines has provided an algorithm for evaluating what preoperative testing patients need. The guidelines were updated in 2002 and can be accessed online athttp://circ.ahajournals.org/cgi/content/full/105/10/1257.29 More recently, another study30 found that patients with stable cardiac disease do not benefit from coronary revascularization before elective vascular procedures. These data were not extrapolated to patients with unstable angina, aortic stenosis, or severe left ventricular dysfunction, and it was postulated that medical optimization before repair may have contributed to improved outcomes. Therefore, preoperative cardiac evaluation, including use of cardiac medications, is appropriate in certain patients who are to undergo elective open AAA repair. The cardiovascular evaluation is intended to reduce perioperative risk and improve long-term survival. There are insufficient data to justify a reduced preoperative cardiology work-up before endovascular repair.31
Patients with chronic obstructive pulmonary disease have a higher risk of major clinical complications from AAA, particularly in the presence of concurrent cardiac disease, suboptimal chronic obstructive pulmonary disease management, or chronic renal disease.32 Smoking cessation for at least two months preoperatively has been found to decrease the risk of pulmonary morbidity compared with patients who smoke up to the time of surgery.33
A study34 of 8,185 intact and 1,829 ruptured AAA repairs showed that impaired renal function has a strong effect on mortality. The mortality rate was 41.2 percent in patients with renal failure and 6.2 percent in patients with normal renal function (P = .001) among patients undergoing elective repair of an intact AAA. Similar results were found in patients undergoing repair of a ruptured AAA.34 Diabetes does not increase mortality following AAA repair, but it is associated with an increase in length of hospital stay.35 Recognition and optimization of a patient’s comorbidities before open AAA repair may reduce postoperative morbidity.
Open vs. Endovascular Repair
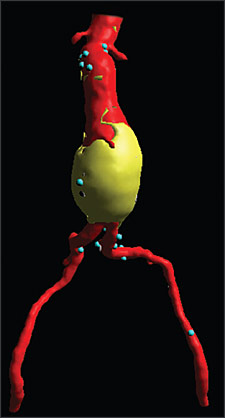
The two primary methods of AAA repair are open and endovascular. Before repair, a CT scan of the aorta and iliac arteries is required (Table 317). Traditional open AAA repair involves direct access to the aorta through an incision in the abdomen. This repair method is well established as definitive, requiring essentially no follow-up radiologic studies. The majority of patients undergoing open AAA repair remain without significant graft-related complications during the rest of their lives (0.4 to 2.3 percent incidence of late graft-related complications in recent studies).36
Endovascular repair of an AAA involves gaining access to the lumen of the abdominal aorta, usually via small incisions over the femoral vessels. An endograft, typically a cloth graft with a stent exoskeleton, is placed within the lumen of the AAA extending distally into the iliac arteries. This serves as a bypass and decreases the pressure on the aortic wall, leading to a reduction in AAA size over time and a decrease in the risk of aortic rupture. Close follow-up is required after endovascular repair with CT scans performed at one, six, and 12 months, and then yearly to ensure that the graft is accomplishing its intended goal (e.g., asymptomatic patient, decreasing AAA size, structurally intact endograft, no fixation site problems or significant graft migration).
Endograft AAA repair was approved by the U.S. Food and Drug Administration in 1999 and it remains a relatively new technology; outcomes greater than five years in patients with endografts are now available.37 In-hospital mortality of AAA open repair is 3.8, versus 1.2 percent for endovascular repair.38 Thirty-day mortality has been reported as 1.1 to 2.7 percent for open repair and 0 to 1.7 percent for endovascular repair37,39; however, a five-year comparison of open versus endovascular repair did not show a significant difference in all-cause mortality during a recent nonrandomized prospective analysis. Postprocedural conversion to an open repair from endovascular was required in 2.8 percent of patients.37 The best evidence is from the Endovascular Aneurysm Repair (EVAR) 1 trial.40 It randomized 543 patients to endovascular repair and 539 to traditional open repair; all of the patients were candidates for open repair. After three years, the all-cause mortality was identical in the two groups (28 percent).40 The EVAR 2 trial41 compared endovascular repair with watchful waiting in patients who were not candidates for open repair. Although 25 percent of patients in the watchful waiting group underwent AAA repair during the four years of follow-up, there was no difference in all-cause mortality between the groups.41
Emergent repair of ruptured AAAs is traditionally performed using the open method. However, more centers are performing endovascular repair on ruptured aneurysms that fit anatomic and physiologic criteria and experiencing promising results.42