
Am Fam Physician. 2006;73(7):1223-1229
Patient information: See related handout on dementia with Lewy bodies, written by the authors of this article.
Author disclosure: Nothing to disclose.
Dementia with Lewy bodies appears to be the second most common form of dementia, accounting for about one in five cases. The condition is characterized by dementia accompanied by delirium, visual hallucinations, and parkinsonism. Other common symptoms include syncope, falls, sleep disorders, and depression. The presence of both Lewy bodies and amyloid plaques with deficiencies in both acetylcholine and dopamine neurotransmitters suggests that dementia with Lewy bodies represents the middle of a disease spectrum ranging from Alzheimer’s disease to Parkinson’s disease. The diagnosis of dementia with Lewy bodies is based on clinical features and exclusion of other diagnoses. Individualized behavioral, environmental, and pharmacologic therapies are used to alleviate symptoms and support patients and their families. Cholinesterase inhibitors are more effective in patients who have dementia with Lewy bodies than in those with Alzheimer’s disease. Conversely, patients who have dementia with Lewy bodies do not respond as well to antiparkinsonian medications. Anticholinergic medications should be avoided because they exacerbate the symptoms of dementia. Traditional antipsychotic medications can precipitate severe reactions and may double or triple the rate of mortality in patients who have dementia with Lewy bodies.
Dementia with Lewy bodies was first described in 1984 by Kosaka and colleagues,1 who reported finding Lewy bodies (i.e., eosinophilic cytoplasmic inclusions in the brainstems of patients affected by Parkinson’s disease) throughout the cortexes of some patients with dementia, rather than the neuritic plaques and neurofibrillary tangles characteristic of Alzheimer’s disease.2 The key features of dementia with Lewy bodies were clarified in the 1996 consensus guidelines3 published after the first international workshop of the Consortium on Dementia with Lewy Bodies (Table 13).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Dementia with Lewy bodies should be distinguished from Alzheimer’s disease by fluctuations in cognitive state, especially daytime drowsiness, daytime sleep of two hours or more, staring into space, and disorganized speech. | C | 17,18 |
| Neuroimaging should not be used to differentiate between dementia with Lewy bodies and Alzheimer’s disease. | C | 12,23 |
| Neuroleptic drugs, especially older agents, should be avoided in patients who have dementia with Lewy bodies because they may cause severe reactions in more than one half of these patients and are associated with increased mortality. | C | 30 |
Central features; required for diagnosis of dementia with Lewy bodies:
|
REM = rapid eye movement.
Adapted with permission from McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1114.
Although understanding of dementia with Lewy bodies is still evolving, family physicians must be able to recognize and appropriately manage this condition; the treatment and prognosis differ from those of Alzheimer’s disease, Parkinson’s disease with dementia, and the vascular dementias. This is especially important because of the severe reaction to neuroleptic medications associated with dementia with Lewy bodies and the greater response to cholinesterase inhibitors.4Epidemiology
Dementia with Lewy bodies is the second most common histopathology found in dementia, exceeded only by Alzheimer’s disease.5 At least 5 percent of noninstitutionalized adults 85 years and older are believed to have dementia with Lewy bodies, and the disease represents approximately 22 percent of all patients with dementia.4 The number of cases is expected to increase as the population ages and as dementia with Lewy bodies is increasingly recognized in the differential diagnosis of dementia. To date, no specific risk factors for dementia with Lewy bodies have been identified.4,6
Pathology
The relationships among dementia with Lewy bodies, Alzheimer’s disease, and Parkinson’s disease with dementia are difficult to define because the current evidence supports different points of view. Some experts believe that dementia with Lewy bodies is related to Alzheimer’s disease or Parkinson’s disease with dementia, but the emerging consensus is that dementia with Lewy bodies is a distinct pathologic entity somewhere between the two.7
Pathologically, classical Alzheimer’s disease is associated with amyloid plaques and neurofibrillary tangles distributed in the parietal, temporal, and parieto-occipital cortex, whereas Parkinson’s disease is associated with Lewy bodies primarily in the subcortical regions of the brain, predominantly in the midbrain substantia nigra and locus ceruleus.8 In contrast, dementia with Lewy bodies is characterized by the presence of Lewy bodies in the subcortical and cortical (frontotemporal) regions of the brain, as well as amyloid plaques. Neurofibrillary tangles are less common in dementia with Lewy bodies.4,8
Recent research has shown that Lewy bodies are eosinophilic cytoplasmic inclusions that contain deposits of a protein called alpha-synuclein.4,7,9 Both Parkinson’s disease with dementia and dementia with Lewy bodies are synucleinopathies, meaning they are neurodegenerative conditions associated with abnormal aggregations of alpha-synuclein.10 Conversely, Alzheimer’s disease is an amyloidopathy with extracellular senile plaques containing beta amyloid and/or a tauopathy with neurofibrillary tangles of the microtubular protein tau.4,8,9 Dementia with Lewy bodies usually presents with both Lewy bodies and senile plaques.
Biochemically, dementia with Lewy bodies is associated with deficits in both acetylcholine and dopamine, which are the primary neurotransmitter deficits in Alzheimer’s disease and Parkinson’s disease, respectively.11 Thus, clinically, pathologically, and biochemically, dementia with Lewy bodies appears to fall somewhere in the middle of a disease spectrum ranging from Alzheimer’s disease to Parkinson’s disease (Figure 1).
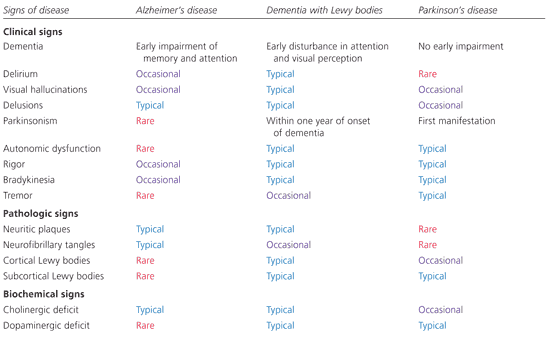
Clinical Features
The overall clinical picture must be considered when making the diagnosis, because neither neuropsychologic testing nor neuroimaging reliably differentiates dementia with Lewy bodies from Alzheimer’s disease,12 and both conditions may exist in a single patient.13 The most widely accepted definition can be found in the consensus guidelines from the international consortium (Table 1).3 A useful acronym to remember the cardinal features of dementia with Lewy bodies is DDaVP: Dementia, Delirium (fluctuating cognition), and Visual hallucinations with Parkinsonism. Some authors include extreme sensitivity to antipsychotic agents,2 whereas others include rapid eye movement (REM) sleep behavior disorder as defining characteristics.14 Each element of this acronym is discussed below.
DEMENTIA
The symptoms of dementia with Lewy bodies can be similar to those of Alzheimer’s disease, making the diagnosis difficult. Patients who have dementia with Lewy bodies tend to have more problems with executive functioning (e.g., planning, prioritizing, sequencing) and to have visuospatial impairment, they but do better with verbal memory than patients with Alzheimer’s disease. Thus, memory changes on the Mini-Mental State Examination may be more prominent with Alzheimer’s disease, whereas difficulties in clock drawing or figure copying may be more indicative of dementia with Lewy bodies.4,14–16
DELIRIUM (FLUCTUATING COGNITION)
Fluctuating cognition occurs in 50 to 75 percent of patients who have dementia with Lewy bodies.4,17 These fluctuations may occur over minutes, hours, or days, and their presence may be particularly helpful in differentiating dementia with Lewy bodies from Alzheimer’s disease.4,15,17,18 The fluctuating cognitive state closely mimics delirium and has been referred to as “pseudodelirium.” In a recent study,18 researchers found four characteristics of fluctuations that significantly differentiated dementia with Lewy bodies from Alzheimer’s disease. These included: (1) daytime drowsiness and lethargy, (2) daytime sleep of two or more hours, (3) staring into space for long periods, and (4) episodes of disorganized speech. The presence of three or four of these features was seen in 63 percent of patients who had dementia with Lewy bodies compared with 12 percent of those with Alzheimer’s disease.18 Input about the patient’s cognitive state should be sought from family members and caregivers.4 Physicians should not rely on clinical impressions at a single visit, because the patient’s cognitive impairment may range from near normal to severe confusion.
VISUAL HALLUCINATIONS
Psychotic symptoms occur in about 80 percent of patients who have dementia with Lewy bodies.3,15 Usually these are purely visual, vivid, colorful, 3-dimensional hallucinations of humans or animals.3,4 Although these hallucinations may upset the family and caretakers, they often are not particularly distressing to the patient.4 Nevertheless, because patients who have dementia with Lewy bodies can experience severe reactions to antipsychotic medications, it is important to recognize these hallucinations as part of the disease spectrum and not as evidence of a superimposed psychotic illness.
PARKINSONISM
In Parkinson’s disease with dementia, the motor symptoms usually predate the dementia by many years. In contrast, to diagnose dementia with Lewy bodies, the onset of dementia and parkinsonism must occur within one year of each other,4 and either feature can be the initial symptom. Although bradykinesia, rigidity, and falls are common to both diseases, tremor often is absent in dementia with Lewy bodies. These patients tend to show an axial bias with greater postural instability, gait difficulty, and facial impassivity, but less tremor than patients who have Parkinson’s disease with dementia.4,12,15 Autonomic symptoms, especially orthostatic hypotension and constipation, typically are prominent in dementia with Lewy bodies.2,14,15 Finally, patients who have dementia with Lewy bodies tend not to respond as well to levodopa with carbidopa (Sinemet) as patients who have Parkinson’s disease with dementia.2,4
OTHER FEATURES
Depression is common in patients who have dementia with Lewy bodies. REM sleep behavior disorder occurs in about one half of these patients and is often a precursor of dementia with Lewy bodies.12,19 In fact, some experts include it as one of the major diagnostic criteria for dementia with Lewy bodies.14 REM sleep behavior disorder is manifested by vivid dreams associated with simple or complex motor behavior during REM sleep.4 Patients act out their dreams, often defending themselves against attack.20,21 The presence of REM sleep behavior disorder in a patient with dementia and depression may be an additional supportive feature for the diagnosis of dementia with Lewy bodies, helping to differentiate this type of dementia from Alzheimer’s disease.20 Autonomic dysfunction (with orthostatic hypotension and carotid-sinus hypersensitivity, as well as postural instability) also is more common in patients who have dementia with Lewy bodies.15 Clinically, autonomic dysfunction may present as dizziness, presyncope, syncope, or falls.4 Urinary incontinence also may appear early in the course of dementia with Lewy bodies and helps to distinguish the condition from Alzheimer’s disease.22
Diagnostic Studies
Unfortunately, dementia with Lewy bodies has no characteristic laboratory findings that distinguish it from other forms of dementia (Figure 2). Appropriate laboratory investigations are identical to those for other forms of dementia and should include routine screening for depression, vitamin B12 deficiency, and hypothyroidism (plus syphilis in persons who may have been infected previously).12 Similarly, although computed tomography (CT) may be indicated as part of the general work-up for dementia, there are no distinctive diagnostic findings of patients who have dementia with Lewy bodies.4,12 Magnetic resonance imaging in dementia with Lewy bodies shows preservation of hippocampal and medial temporal lobe volume compared with Alzheimer’s disease, whereas single photon emission CT reveals occipital hypoperfusion.4 Although highly specialized neuroimaging may be diagnostically useful in the future, present imaging modalities are not sufficiently specific to reliably diagnose dementia with Lewy bodies.15,23
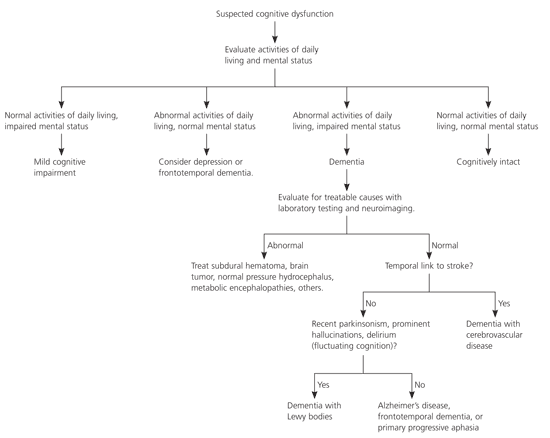
Treatment
Accurate diagnosis, nonpharmacologic interventions, and pharmacologic treatments are all important aspects of the management of dementia with Lewy bodies. Developing a problem list of cognitive, psychiatric, and motor disabilities may be helpful. Working with the patient and family, the family physician can help prioritize target symptoms such as extrapyramidal motor features; cognitive impairment; neuropsychiatric features (e.g., hallucinations, depression, sleep disorder, associated behavioral disturbances); or autonomic dysfunction. Identifying the most disabling and distressing symptoms is especially helpful because treatment in one area may be at the expense of losses in another.4 For example, use of cholinesterase inhibitors for dementia may exacerbate parkinsonian features such as drooling and postural instability.
NONPHARMACOLOGIC TREATMENT
No studies have examined the impact of nonpharmacologic interventions in dementia with Lewy bodies,4 but a wide range of interventions have been beneficial in Alzheimer’s disease and other forms of dementia.15,24–26 These include improving sensory impairment with glasses or hearing aids, educating the patient and family, structuring the environment, and teaching behavioral interventions. The interventions should be selected to address the specific needs of each patient and his or her caregivers. Hallucinations may be managed by benign neglect and caregiver education, as well as by environmental changes such as improving vision and lighting and increasing the number of persons in the environment.2
PHARMACOLOGIC TREATMENT
Pharmacologic management of dementia with Lewy bodies can be challenging (Table 2). In patients with acute psychotic symptoms, other causes of symptoms such as pain, intercurrent infection, subdural hematoma, or adverse drug reaction must be excluded.24 Differentiating pseudodelirium caused by dementia with Lewy bodies from a superimposed delirium can be difficult, and a diagnostic evaluation is still necessary for any abrupt increase in confusion.25,26 Patients with significant visual hallucinations are reported to have better response to cholinesterase inhibitor therapy than other patients with dementia26; these medications improve fluctuating cognition, hallucinations, apathy, anxiety, and sleep disturbances.4,15,24
| Symptom | Preferred medication | Medications to avoid | Goal of therapy | Possible adverse effects |
|---|---|---|---|---|
| Delirium | Cholinesterase inhibitors | Antipsychotics (especially neuroleptics with high D2 affinity, such as haloperidol [Haldol]) | Decrease fluctuations in cognition | GI symptoms, rare worsening of EPS |
| Dementia | As above | As above | Improve cognition | As above |
| Parkinson’s disease symptoms | Levodopa with carbidopa (Sinemet) | Anticholinergics, antimuscarinics | Improve mobility | Visual hallucinations, delusions, orthostatic hypotension, GI upset |
| REM sleep behavior disorder | Clonazepam (Klonopin) | None | Improve sleep | Ataxia, morning sedation |
| Visual hallucinations | Cholinesterase inhibitors | Antipsychotics (especially neuroleptics with high D2 affinity, such as haloperidol) | Reduce number of hallucinations | GI symptoms, rare worsening of EPS |
The results of a trial27 of rivastigmine (Exelon) compared with placebo in 120 patients reported statistically and clinically significant behavioral effects at 20 weeks. According to a Cochrane review,28 patients who have dementia with Lewy bodies and behavioral or psychiatric problems may benefit from rivastigmine if they tolerate it, but the evidence is weak. In addition to gastrointestinal (GI) side effects, hypersalivation, postural hypotension, and falls may increase when cholinesterase-inhibiting drugs are used.
Patients who have dementia with Lewy bodies should not be given the older, typical D2-antagonist antipsychotic agents such as haloperidol (Haldol), fluphenazine (Prolixin), and chlorpromazine (Thorazine).4,15 Patient records should document this and caregivers should be informed. As many as one half of patients who have dementia with Lewy bodies who receive neuroleptic medications experience life-threatening adverse effects characterized by sedation, rigidity, postural instability, falls, increased confusion, and neuroleptic malignant syndrome, with an associated two- to threefold increase in mortality.4,15,24,29,30 Atypical antipsychotics may be tried in low doses, but these can cause similar adverse effects and increase the risk of stroke.31
If antiparkinsonian drugs are prescribed, the lowest possible dose of levodopa with carbidopa should be used, and monotherapy is preferred.4,15 The effect on parkinsonian symptoms is probably less than in classic Parkinson’s disease,4,15 and potential side effects include visual hallucinations, delusions, orthostatic hypotension, and GI upset.15 The goal of antiparkinsonian medication is to improve mobility without inducing or exacerbating psychotic symptoms or confusion.
Information is not yet available about the use of cholinesterase inhibitors in combination with antiparkinsonian or atypical antipsychotic medications in dementia with Lewy bodies, although this is a common clinical situation.24 Anticholinergic agents should be strictly avoided. Orthostatic hypotension can be treated with vigorous hydration, ample dietary sodium, avoidance of prolonged bed rest, efforts to stand up slowly, and avoidance of medications that contribute to orthostasis. Constipation can be treated with exercise, increased dietary fiber, and hydration.
The diagnosis of REM sleep behavior disorder often is based on complaints from the patient’s bed partner. This disorder responds to clonazepam (Klonopin), 0.25 to 1 mg at bedtime, although use of this drug may be limited by ataxia and morning sedation.2,20 Management of the REM sleep behavior disorder is reported to improve fluctuations in cognition and markedly benefit quality of life for patients who have dementia with Lewy bodies and their families.4
Prognosis
Considerable uncertainty exists about the progression and survival of patients who have dementia with Lewy bodies. Most experts believe that the rate of decline and mortality in dementia with Lewy bodies is similar to that of Alzheimer’s disease,32 but some studies indicate shorter survival for patients who have dementia with Lewy bodies.33 No factors that predict a more severe clinical course or decreased survival have been identified.4