
Am Fam Physician. 2006;73(12):2162-2168
A more recent article on hepatitis A is available.
Patient information: See related handout on hepatitis A.
Author disclosure: Nothing to disclose.
The introduction of hepatitis A vaccines in 1995 led to a drop in the number of reported cases of hepatitis A and a shift to a higher percentage of cases occurring in older age groups. The hepatitis A virus survives for extended periods in the environment. Transmission primarily is fecaloral, although there have been rare instances of transmission through blood products. The virus appears sporadically and is spread by close personal contact, with occasional food-borne outbreaks. Older persons infected by the virus usually develop a symptomatic infection with abrupt onset, fever, and jaundice lasting two months. Children usually have an asymptomatic infection and rarely develop jaundice. Laboratory diagnosis is made by detection of antihepatitis A virus immunoglobulin M in serum. Ten to 20 percent of symptomatic patients experience a prolonged or relapsing course of illness, but chronic infection has not been reported. Fulminant infection occurs in less than 1 percent of patients and can result in emergent liver transplant or death. Prevention starts with thorough handwashing and careful food handling. Prompt disease reporting, the identification of exposed persons, and expeditious administration of immune globulin prevent secondary transmission of the disease. Physicians should consider routine vaccination of children 12 to 23 months of age based on recommendations from the Centers for Disease Control and Prevention. Vaccination for children two years or older and adults should be included in routine preventive care for those at increased risk of contracting the disease (e.g., travelers to certain countries, men who have sex with men, drug abusers, recipients of clotting factor replacement) and for persons with chronic liver disease.
Targeted use of hepatitis A vaccines in the United States since 1995 has led to a dramatic decrease in the number of reported cases of hepatitis A, from 32,000 in 1990 to 7,700 in 2003,1 with most of the decrease occurring in children.2 As a result of this trend and new cost-effectiveness data, the Advisory Committee on Immunization Practices (ACIP) has recommended vaccination against hepatitis A virus for all children during routine immunization at the age of 12 to 23 months.3 With the strategy of universal vaccination, the disease could potentially be eradicated in the United States.
Even before the targeted use of the vaccine, good sanitation practices resulted in a generally low incidence of hepatitis A in the United States, with a correspondingly low overall immunity rate of about 33 percent.4 This low population immunity creates the potential for epidemi cs of symptomatic disease resulting from food- or water-borne transmission, such as that which occurred in four eastern states in 2003 caused by imported contaminated raw green onions in restaurant salsa.5
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| The antihepatitis A virus immunoglobulin M test should be used as a confirmatory test only in individuals with signs and symptoms suggestive of hepatitis A. | C | 18 |
| Caregivers of patients who are diapered or incontinent should take contact precautions for the first two weeks of illness, but precautions are not recommended for more than one week after the onset of jaundice in adults. Prolonged enteric precautions are recommended when the patient is a child. | C | 10,11 |
| Persons who have not been immunized and who have been in close personal contact with a patient with acute hepatitis A should receive postexposure prophylaxis with immune globulin within two weeks after the last exposure. | C | 9–11 |
| Administration of hepatitis A vaccine is recommended for travelers to certain countries outside the United States, users of illicit drugs, men who have sex with men, persons with clotting factor disorders, persons with chronic hepatitis, and some laboratory workers. It is not recommended routinely for those who work in health care, day care, or food service, or for workers who are exposed to sewage. | B | 7,9,11,25 |
| Administration of hepatitis A vaccine is recommended as part of routine childhood immunizations between ages one and two years. | B | 3 |
Viral Characteristics and Epidemiology
Hepatitis A is caused by a nonenveloped RNA picornavirus that infects only primates. Lack of a lipid envelope confers resistance to bile lysis.6 The virus is hardy, surviving on human hands and fomites and requiring temperatures higher than 185°F (85°C) for inactivation.4,7 Hepatitis A virus survives for extended periods in seawater, fresh water, wastewater, and soil.4,8 The virus is resistant to freezing, detergents, and acids, but it is inactivated by formalin and chlorine.8
Infection occurs primarily by oral inoculation of fecally excreted virus either by person-to-person contact (including any form of sexual contact with proximity to feces) or by ingestion of contaminated food or water.8–11 Viral particles are replicated only in hepatocytes and gastrointestinal epithelial cells and are released into blood and bile by a mechanism that does not cause cell lysis.4 Liver cells are destroyed by a cell-mediated immune response.4,6,8
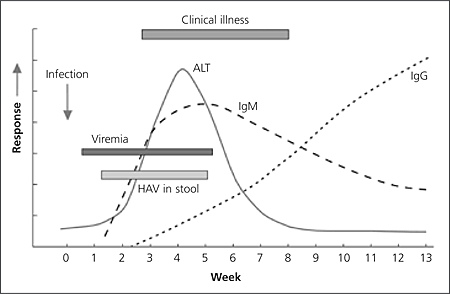
The incubation period is 15 to 50 days, with an average of 25 to 30 days.4,6,7,10–12 Peak infectivity correlates with the greatest viral excretion in the stool during the two weeks before the onset of jaundice or elevation of liver enzyme levels.7 Viremia occurs soon after infection and persists through the period of liver enzyme elevation.9 On rare occasions, hepatitis A virus has been transmitted by transfusion of blood products collected during the donor’s viremic phase.4,6–10 The potential for transmission via intravenous drug use is unknown.6,8
The presence and severity of symptoms with hepatitis A virus infection is related to the patient’s age.7 Approximately 70 percent of infected adults develop symptoms, including jaundice.4,9,11 In contrast, only 30 percent of children younger than six years of age develop symptoms, which usually are nonspecific and flu-like without jaundice.9 Because even asymptomatic infected children may shed virus in their stools for up to six months, infection in children often initiates and perpetuates community-wide outbreaks.7
The 7,700 cases of hepatitis A reported to the Centers for Disease Control and Prevention (CDC) in 2003 met the case definition of an acute illness with discrete onset of symptoms, jaundice, or elevated serum transaminase levels, and a positive antihepatitis A virus immunoglobulin M (IgM) result.7 Adjusting for underreporting and asymptomatic infections, the estimated number of new infections was 61,000, approximately one third that of 1999.13
The source of most reported food-borne outbreaks in the United States is infected food handlers at the point of sale,10 followed by fresh produce, particularly green onions and strawberries.14,15 Consumption of shellfish harvested from contaminated waters is a significant cause outside the United States.10,15 Studies performed in the United States have not shown an occupational risk for health care, child care, or food service workers or workers exposed to sewage.7,9
Diagnosis
Risk factors for hepatitis A and other causes of acute hepatitis should be elicited in the history. Case reports have identified the following risk factors for hepatitis A: close personal contact with an infected person (17 percent of cases), men who have sex with men (17 percent), travel outside the United States (10 percent), illicit drug use (8 percent), known food-borne outbreak (6 percent), and contact with a child or employee in a child care center (5 percent).17 In about one half of reported cases, no risk factor was identified.7
Initial diagnostic tests include determination of hepatic enzyme and bilirubin levels with follow-up viral serology for hepatitis A, B, and C. In patients with hepatitis A, serum transaminase levels may reach 10,000 U per L or higher, but there is little correlation between level and disease severity.4 The alkaline phosphatase level usually is elevated only minimally.6 The bilirubin level usually is elevated to about 5 to 10 mg per dL (86 to 171 μmol per L), and prothrombin time usually is 11 to 26 seconds.4
The antihepatitis A virus IgM test is the preferred confirmatory test for acute hepatitis A because it has high sensitivity and specificity when used on specimens from persons with typical symptoms.4,18 However, its use among persons without symptoms of hepatitis produces a high percentage of false-positive results, which may lead to unnecessary public health investigations.18
Serum antihepatitis A virus IgM usually can be detected five to 10 days before symptom onset, and the level remains elevated for four to six months.7,9,11,16 The antihepatitis A virus IgG level begins to rise soon after the IgM level, and antihepatitis A virus IgG is present throughout the person’s lifetime, conferring immunity (Figure 116).7,9,16
Clinical Course
More than 80 percent of adults with hepatitis A are ill for up to eight weeks6 and miss about 30 days of work.8,9 The preicteric phase lasts five to seven days, with abrupt onset of fever, malaise, anorexia, nausea, vomiting, abdominal pain, and headache. Less common symptoms include chills, myalgias, arthralgias, cough, diarrhea, constipation, pruritus, and urticaria.4 Physical signs include tender hepatomegaly, splenomegaly, bradycardia, and posterior cervical lymphadenopathy.4,6 The icteric phase, which lasts four to 30 days, begins with conjugated bilirubinuria followed within a few days by pale, clay-colored stools and jaundice.6 Chronic infection does not occur.11
Complications
| Gastrointestinal findings | |
| Acalculous cholecystitis | |
| Pancreatitis | |
| Hematologic findings | |
| Aplastic anemia | |
| Autoimmune hemolysis | |
| Autoimmune thrombocytopenic purpura | |
| Hemolysis (in patients with glucose-6-phosphate dehydrogenase deficiency) | |
| Red cell aplasia | |
| Neurologic findings | |
| Guillain-Barré syndrome | |
| Mononeuritis | |
| Mononeuritis multiplex | |
| Postviral encephalitis | |
| Transverse myelitis | |
| Renal findings | |
| Acute tubular necrosis | |
| Interstitial nephritis | |
| Mesangial proliferative glomerulonephritis | |
| Nephrotic syndrome | |
| Other findings | |
| Cutaneous vasculitis | |
| Cryoglobulinemia | |
| Reactive arthritis | |
Less than 1 percent of patients experience a fulminant course of illness characterized by worsening jaundice and development of encephalopathy. Advanced age and comorbid conditions such as chronic liver disease increase the risk of a fulminant course, which often results in death or an emergent liver transplant.4,6 Prognostic indicators used to support the need for a liver transplant are age younger than 10 years or older than 40 years, jaundice lasting more than seven days before the onset of encephalopathy, increased levels of serum bilirubin (more than 17 mg per dL [291 μmol per L]), and prolonged prothrombin time (more than 25 seconds).19 The overall fatality rate is relatively low (0.3 percent), but increases to 2 percent in adults older than 40 years.7
Treatment
Treatment is supportive and includes appropriate rest when necessary,4 balanced nutrition, and avoidance of hepatotoxins such as alcohol and acetaminophen.6 No specific antiviral therapy currently is available.8,12 About 30 percent of symptomatic patients require hospitalization for dehydration, severe prostration, coagulopathy, encephalopathy, or other evidence of hepatic decompensation.6,17
Caregivers should observe strict contact precautions during the infectious period with patients who are diapered or incontinent. Otherwise healthy adult patients are noninfectious by two weeks after the onset of illness, but children and immunocompromised persons may remain infectious for up to six months.8–11
Immune Globulin
Immune globulin administered intramuscularly provides short-term protection (i.e., three to five months) through passive transfer of hepatitis A virus antibody. Dosage information is given in Table 3.7,9,11,20 The immune globulin is made from pooled human plasma that has been treated to inactivate viruses and that tested negative for human immunodeficiency virus and hepatitis B and C. No transmission of viral infection has been reported, and serious adverse reactions are rare.
Administration of immune globulin is not contra-indicated during pregnancy or lactation. Known IgA deficiency is a contraindication because there have been reports of anaphylaxis after repeated intramuscular administration of immune globulin.9
Immune globulin administered intramuscularly as prophylaxis within two weeks after exposure to hepatitis A virus is 69 to 89 percent effective in preventing symptomatic infection.4,21 When infection is not prevented, immune globulin attenuates symptoms and reduces further viral transmission.22 Effectiveness when administered before exposure is 80 to 85 percent.21
Administration of intramuscular immune globulin should be avoided within two to three weeks after administration of live, attenuated vaccines because it decreases their immunogenicity. Administration of these vaccines should be delayed until three months (for measles, mumps, and rubella vaccinations) or five months (for varicella vaccination) after intramuscular immune globulin administration.7,9
Vaccine
Two types of inactivated whole virus vaccines for hepatitis A virus were introduced in the United States in 1995 and 1996. Dosages and schedules are given in Table 3.7,9,11,20 The first dose provides immunity in 37 percent of persons within two weeks, in 90 percent of persons within four weeks, and in 100 percent of persons at 26 weeks.22 The second dose provides persistent immunity projected to last at least 20 years.7,9 The vaccines are interchangeable,22 and both are contraindicated in persons with a history of serious allergic reaction to a vaccine component or a previous dose.7
Prevention Methods
Prevention methods include sanitation, case investigation with contact postexposure prophylaxis, and primary vaccination. Physicians should instruct patients about thorough handwashing after defecation and diaper changing, and sanitary disposal of wastes. Ensuring careful food-handling practices, particularly of produce and shellfish, are public health focuses.5
Prompt reporting to the local health department will trigger an investigation to determine the source of the infection, identify exposed persons, and provide postexposure prophylaxis. Disease reporting is required of physicians, laboratories, and school officials. Previously unimmunized contacts of a patient with acute hepatitis A should receive intramuscular immune globulin without a delay for serologic testing (Table 4).9–11 Hepatitis A vaccine may be given simultaneously with intramuscular immune globulin if the vaccine otherwise would be recommended for that person according to his or her risk status.10 One study23 showed hepatitis A vaccine given alone to have a protective efficiency of 79 percent in preventing transmittal of hepatitis A virus in household and personal contacts when administered within eight days of exposure. However, the study was small and did not involve a comparison with intramuscular immune globulin,15,20 thus vaccine alone is not currently recommended for post-exposure prophylaxis.11,15 Community-wide vaccination of children has proved effective in controlling localized outbreaks.9,24
| Postexposure prophylaxis should be considered in persons who: |
| Live in the same house as the patient, share a room in a residential care facility with the patient, or care for the patient’s personal needs in a residential care facility |
| Engaged in sexual relations or other intimate contact with the patient or shared illicit drugs with the patient |
| Work with the patient in a food service establishment where each person handled food, or patronized a restaurant in which the patient handled foods served without further cooking during the time the patient was infectious0 |
| Work at or attend a child care center in the same classroom as the patient if all children in the facility are toilet-trained |
| Work at or attend a child care center in which some children are not toilet trained and hepatitis A has been diagnosed in an employee, in a child, or in household contacts of two or more enrolled children |
| Work at or attend school or a health care setting in which multiple persons with hepatitis A virus have been identified |
| Postexposure prophylaxis is not recommended in: |
| Casual contacts of the patient |
| Hospital staff members |
| Persons who have received one dose of hepatitis A vaccine at least one month before exposure |
| Persons with an immunoglobulin A deficiency (contraindicated) |
| Persons in the same school, work, or health care setting in which only one case of hepatitis A has been identified, unless otherwise indicated in recommendations above |
Routine health maintenance visits should include an assessment of risk factors for hepatitis A and appropriate vaccination. Hepatitis A vaccine is recommended for persons who have a higher risk of hepatitis A virus infection (e.g., travelers to certain countries outside the United States [see below], illicit drug users, men who have sex with men, persons receiving clotting factor concentrates). Vaccination also is recommended for persons with chronic liver disease because of the higher risk of mortality. Routine vaccination is not recommended for persons at risk of occupational exposure,7,9,11 but it should be considered in certain situations (e.g., research laboratory workers studying hepatitis A virus).25 In 1999, the ACIP recommended that children living in the 17 western states with incidence rates above the national average be vaccinated routinely or considered for vaccination.9 Vaccination is mandated for school entry in Alaska, Arizona, Nevada, New Mexico, Oklahoma, Texas, and Utah.26 In October 2005 (published in May 2006), the ACIP recommended that universal hepatitis A vaccination be implemented and integrated into the routine childhood immunization schedule at age one to two years. This recommendation was included in the CDC immunization schedule in January 2006.3
Prophylaxis is recommended for persons traveling to any part of the world except Canada, western Europe, Japan, New Zealand, and Australia. Intramuscular immune globulin may be used in children younger than one year, but hepatitis A vaccine is preferred for persons one year and older.11 If the departure is scheduled within four weeks after vaccination, intramuscular immune globulin should be administered concomitantly.9,27
Testing for immunity with total antihepatitis A virus level before vaccination is cost-effective only in persons who have a high likelihood of immunity from previous hepatitis A virus infection.9 In the United States, where increases with age, testing could be considered in persons older than 40 years, persons with a history of jaundice, and persons who lived in endemic areas as children.7,11