
Am Fam Physician. 2006;74(4):619-626
Patient information: See related handout on rickets, written by the authors of this article.
Author disclosure: Nothing to disclose.
Rickets develops when growing bones fail to mineralize. In most cases, the diagnosis is established with a thorough history and physical examination and confirmed by laboratory evaluation. Nutritional rickets can be caused by inadequate intake of nutrients (vitamin D in particular); however, it is not uncommon in dark-skinned children who have limited sun exposure and in infants who are breastfed exclusively. Vitamin D–dependent rickets, type I results from abnormalities in the gene coding for 25(OH)D3-1-α-hydroxylase, and type II results from defective vitamin D receptors. The vitamin D–resistant types are familial hypophosphatemic rickets and hereditary hypophosphatemic rickets with hypercalciuria. Other causes of rickets include renal disease, medications, and malabsorption syndromes. Nutritional rickets is treated by replacing the deficient nutrient. Mothers who breastfeed exclusively need to be informed of the recommendation to give their infants vitamin D supplements beginning in the first two months of life to prevent nutritional rickets. Vitamin D–dependent rickets, type I is treated with vitamin D; management of type II is more challenging. Familial hypophosphatemic rickets is treated with phosphorus and vitamin D, whereas hereditary hypophosphatemic rickets with hypercalciuria is treated with phosphorus alone. Families with inherited rickets may seek genetic counseling. The aim of early diagnosis and treatment is to resolve biochemical derangements and prevent complications such as severe deformities that may require surgical intervention.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Physicians should provide 200 IU of vitamin D per day to all breastfed and nonbreastfed infants who consume less than 500 mL of vitamin D–fortified formula per day and all children and adolescents who consume less than 500 mL of vitamin D–fortified milk per day, do not get regular sunlight exposure, and do not get 200 IU of vitamin D supplement per day from a multivitamin. | C | 12,20 |
| Surgical intervention may be necessary to repair severe bony abnormalities in children with rickets, but it should not be undertaken until the biochemical derangements have resolved so that optimal healing occurs at the surgical site. | C | 31 |
| Vitamin D–deficiency rickets can be treated initially with high-dose vitamin D and calcium and phosphorus supplements. | C | 35–37 |
Illustrative Case
The mother of a 26-month-old black infant expresses concern that her son is not growing properly. Born at 34 weeks of gestation at 3 lb, 5 oz (1.5 kg), he was exclusively breastfed until 11 months of age. He is a picky eater, is breastfed twice daily, and consumes minimal dairy products.
His examination reveals a weight of 21 lb (9.6 kg; below the fifth percentile for age), height of 72.5 cm (below the fifth percentile), and head circumference of 74.5 cm (50th percentile). Other abnormal findings include frontal bossing, open anterior fontanel, wide wrists, and bowlegs.
Nutritional rickets is suspected and confirmed with laboratory and radiographic evaluations. Levels of serum alkaline phosphatase and parathyroid hormone are elevated, and calcidiol (25[OH]D3) is decreased. Radiographic images of the wrist show fraying and cupping of the distal radius and ulna, as well as bone demineralization. Treatment is initiated with oral solution of ergocalciferol at 4,000 IU per day for six weeks with close monitoring of calcium and phosphorus levels.
Introduction
Rickets is not a disease only of the past, nor is it limited to developing countries. Until recently, vitamin D supplementation for breastfed infants was not advised.1,2 However, multiple case reports3–11 of nutritional rickets in the United States have prompted the recent recommendation by the American Academy of Pediatrics to provide a daily vitamin D supplement for all solely breastfed babies beginning in the first two months of life.12 In the United States, rickets should be included in the differential diagnosis of children presenting with failure to thrive, developmental delay, and orthopedic abnormalities. Early diagnosis is essential because morbidity can be minimized if children are treated before eight months of age.7 An extensive literature search of MEDLINE forms the basis of this report, which briefly addresses several causes of rickets with a more in-depth review of nutritional rickets.
Epidemiology
Because rickets is not a reportable disease in the United States, national data are unavailable. Statistics from Connecticut reveal that less than one third of children with rickets between 1986 and 2000 had nutritional deficiencies, whereas the remaining children had underlying disease or genetic factors responsible for the illness.3 Nutritional rickets is the main type reported outside the United States, followed by vitamin D–dependent, vitamin D–resistant, and renal rickets13 (Table 14,14–20).
| Type | Causes | Inheritance pattern | Clinical features | Treatment* |
|---|---|---|---|---|
| Nutritional rickets or vitamin D–deficiency rickets | Vitamin D deficiency, phosphorus or calcium deficiency (rare), inadequate sunlight exposure, secondary to malabsorption syndromes (IBD, celiac disease, cystic fibrosis [rarely]) | NA | Skeletal findings, abnormal gait, hypocalcemic tetany/seizures, developmental delay, failure to thrive | Replace the deficient nutrient orally; may need to administer vitamin D intramuscularly if rickets secondary to malabsorption. |
| Vitamin D–dependent rickets | ||||
| Type I or pseudovitamin D–deficiency rickets | Deficiency of renal 25(OH) D3–1-α-hydroxylase | Autosomal recessive | Younger than two years, hypocalcemic tetany, severe bony changes, seizures | Calcitriol (Rocaltrol) |
| Type II or hereditary 1-α, 25-dihydroxyvitamin D–resistant rickets | Defective interaction between calcitriol and receptor | Autosomal recessive | Younger than one year, severe bony changes, alopecia | Massive doses of calcitriol and calcium |
| Vitamin D–resistant rickets | ||||
| Familial hypophosphatemic rickets or X-linked hypophosphatemic rickets | Impaired proximal renal tubular reabsorption of phosphorus and inappropriately normal calcitriol levels | X-linked dominant | Short stature, leg bowing, dental abnormalities | Oral phosphate and calcitriol |
| Hereditary hypophosphatemic rickets with hypercalciuria | Impaired proximal renal tubular reabsorption of phosphorus and increased calcitriol | Autosomal recessive, autosoma dominant | Bone pain, muscular weakness | Oral phosphate |
| Miscellaneous | ||||
| Renal rickets or renal osteodystrophy | Loss of functional renal parenchyma caused by chronic renal failure leads to mineral derangements and decreased calcitriol production | NA | Bone pain, arthralgias, fractures, muscle weakness, failure to thrive | Vitamin D and phosphate- binding compound |
| Rickets of prematurity | Multifactorial | NA | Osteopenia, fractures | Replace dietary deficiencies and minimize iatrogenic causes. |
| Tumor-induced or oncogenic rickets | Tumor-induced inhibition of renal 25(OH)D3–1-α-hydroxylase | NA | Fractures, bone pain, muscle weakness | Treat underlying malignancy. |
Unlike developing countries, the United States saw the eradication of nutritional rickets in the 1930s following the discovery that vitamin D possessed antirachitic properties.21 Today, in the absence of ongoing national surveillance, it is difficult to know how likely it is that a child with rickets will present to the primary care physician’s office. As a result, the true burden of this condition must be estimated. In one study,22 the prevalence of nutritional rickets was estimated to be nine cases per 1 million children, whereas the Centers for Disease Control and Prevention places this rate at five cases per 1 million children six months to five years of age.23 Of note, in multiple studies, most affected children were black.22
Pathogenesis
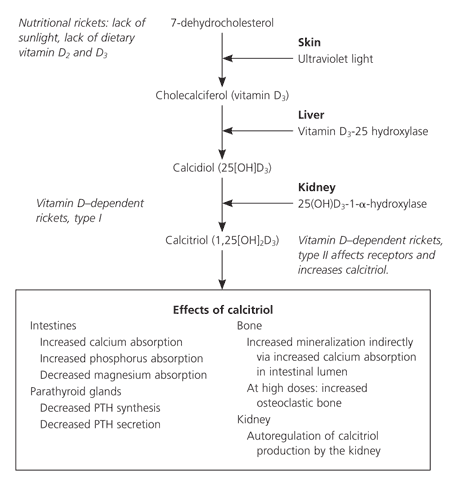
A disease that occurs during childhood, rickets is the failure of growing bone to mineralize. Many skeletal and radiographic changes can occur (Table 214,19,24) because of the lack of calcified osteoid and the buildup of unossified cartilage.14 Proper bone formation requires a complex interplay of several organs and chemicals (Table 3),25 and vitamin D deserves special mention because any disturbance in its production, absorption, or metabolism is paramount in the development of rickets. Human beings maintain adequate levels of vitamin D by producing it from cholesterol or by absorbing it from ingested food sources. Sunlight is a vital component necessary for the production of vitamin D, which begins in the skin and ends in the kidney, as depicted in Figure 1.25
| Bowing or widening of physis | |
| Costochondral beading (rachitic rosary) | |
| Craniotabes | |
| Delayed closure of anterior fontanel | |
| Dental abnormalities | |
| Flaring of ribs at diaphragm level (Harrison’s groove) | |
| Flaring of wrists | |
| Fractures | |
| Fraying and cupping of metaphysis | |
| Frontal bossing of skull | |
| Genu valgum or varum | |
| Lordosis/kyphosis/scoliosis | |
| Osteopenia |
| Chemical | Function |
|---|---|
| Alkaline phosphatase | Exact function unknown; isoenzyme is elevated in conditions such as rickets that are associated with high bone turnover |
| Calcitonin | Bone: inhibits resorption |
| Intestine: inhibits calcium and phosphorus absorption | |
| Kidney: increases calcium excretion, inhibits production of calcitriol | |
| Calcitriol | Bone: indirectly stimulates bone synthesis via increased calcium absorption in intestinal lumen |
| Intestine: increases calcium, phosphorus, and magnesium absorption | |
| Kidney: autoregulation of calcitriol production by the kidney | |
| Parathyroid gland: negative feedback to decrease secretion of parathyroid hormone | |
| Parathyroid hormone | Bone: mobilizes calcium and phosphorus |
| Intestine: indirectly increases calcium and phosphorus absorption by increasing calcitriol | |
| Kidney: increases calcitriol, increases calcium reabsorption, decreases phosphorus reabsorption |
Types of Rickets
NUTRITIONAL
Nutritional rickets results from inadequate sunlight exposure or inadequate intake of dietary vitamin D, calcium, or phosphorus. Although uncommon in the United States, vitamin D deficiency still can occur, particularly when an infant is solely breastfed, is dark skinned, or has limited sunlight exposure. Dark-skinned persons require more sunlight exposure than others to produce the same amount of vitamin D because melanin acts as a neutral filter and absorbs solar radiation.8 A diet deficient in calcium,3 such as one dependent on nonfortified milk substitutes, can lead to rickets.6,10,23 Nutritional rickets presents in the first two years of life with short stature, gait abnormality, developmental delay, and characteristic findings (Tables 14,14–20 and 214,19,24). Commonly, infants younger than six months present with hypocalcemic tetany or seizures, whereas older children present with failure to thrive or skeletal deformities.14
VITAMIN D DEPENDENT
Vitamin D–dependent rickets, type I is secondary to a defect in the gene that codes for the production of renal 25(OH)D3–1-α-hydroxylase (Figure 125). Vitamin D–dependent rickets, type II is a rare autosomal disorder caused by mutations in the vitamin D receptor. Type II does not respond to vitamin D treatment; elevated levels of circulating calcitriol differentiate this type from type I.
VITAMIN D RESISTANT
Rickets refractory to vitamin D treatment may be caused by the most common heritable form, known as vitamin D–resistant rickets or familial hypophosphatemic rickets.15,16 Because of mutations of the phosphate-regulating gene on the X chromosome, renal wasting of phosphorus at the proximal tubule level results in hypophosphatemia.17 Normal levels of calcitriol are found in this disorder.
Hypophosphatemia also can occur secondary to hereditary hypophosphatemic rickets with hypercalciuria, which is believed to result from an isolated defect in renal reabsorption of phosphorus.
OTHER CAUSES
Various medical conditions and medications can cause rickets (Table 4).17,26,27 In rickets secondary to malignancy, the most common pathophysiology is tumor secretion of a renal phosphate–wasting factor and impaired calcitriol production.17 Rickets caused by renal disease (renal osteodystrophy) is caused by disturbances in calcium and phosphorus regulation and calcitriol production. Malabsorption syndromes such as celiac disease and cystic fibrosis can cause vitamin D deficiency.
| Medications | ||||
| Antacids | ||||
| Anticonvulsants | ||||
| Corticosteroids | ||||
| Loop diuretics | ||||
| Malignancy | ||||
| Prematurity | ||||
| Diseases of organs associated with vitamin D and calcium metabolism | ||||
| Kidney disease | ||||
| Liver and biliary tract disease | ||||
| Malabsorption syndromes | ||||
| Celiac disease | ||||
| Cystic fibrosis (rare) | ||||
Premature infants are at risk of developing rickets from calcium and phosphorus deficiency and side effects of their medications (e.g., loop diuretics, corticosteroids). Other medications associated with the development of rickets include anticonvulsants and antacids. Phenytoin (Dilantin) may cause target organ resistance to calcitriol.26 Excess oral administration of aluminum-containing antacids can lead to hypophosphatemic rickets caused by the phosphate-binding property of aluminum.27
Evaluation
MEDICAL HISTORY
The infant’s gestational age, diet, and degree of sunlight exposure should be noted. A detailed dietary history should include specifics of vitamin D and calcium intake. Researchers have suggested an appropriate amount of sunlight exposure for infants (i.e., 30 minutes per week if only in a diaper and two hours per week if fully clothed),28 but the exact amount needed for a particular child is not known. It helps to know if the child avoids sun exposure completely. A family history of short stature, orthopedic abnormalities, poor dentition, alopecia, and parental consanguinity may signify inherited rickets.
The review of systems should focus on growth and orthopedic concerns and signs and symptoms of hypocalcemia, such as muscle cramps, numbness, paresthesias, tetany, and seizures.
PHYSICAL EXAMINATION
In children with rickets, complete physical and dental examinations should be performed. The entire skeletal system must be palpated to search for tenderness and bony abnormalities (Table 214,19,24). Bowlegs in the absence of other findings are relatively common in normal children in the first two years of life; rickets should be suspected in older bowlegged children and in cases associated with asymmetry, pain, or progression in severity. Gait disturbances in the ambulatory child and neurologic abnormalities (such as hyperreflexia) in all children should be sought.
LABORATORY AND RADIOGRAPHIC FINDINGS
Laboratory investigation may include serum levels of calcium (total and ionized with serum albumin), phosphorus, alkaline phosphatase, parathyroid hormone, urea nitrogen, creatinine, and calcidiol. Urine studies include urinalysis and levels of urinary calcium and phosphorus. The serum level of calcidiol is indicative of the patient’s overall vitamin D status.29 Although calcitriol is the active form of vitamin D, it has a short half-life and circulates at a concentration that is 1,000 to 2,000 times less than calcidiol.29 Depending on the stage of the disease, laboratory values can vary. The most common laboratory findings in nutritional rickets are decreases in serum calcium, serum phosphorus, calcidiol, calcitriol, and urinary calcium. Conversely, parathyroid hormone, alkaline phosphatase, and urinary phosphorus levels are elevated.
An anteroposterior radiograph of rapidly growing skeletal areas, such as the knee or wrist, is most helpful in diagnosing rickets. The skeletal changes caused by rickets usually are most pronounced at the knees, wrists, and anterior rib ends (rachitic rosary)24 (Figures 2 through 4). Classic radiographic findings include widening of the distal physis, fraying and widening of the metaphysis, and angular deformities of the arm and leg bones. Bony changes of rickets may be mistaken for other conditions of childhood such as congenital syphilis, osteogenesis imperfecta, or child abuse,30 and it can be beneficial to consult an experienced children’s radiologist for radiographic interpretation.
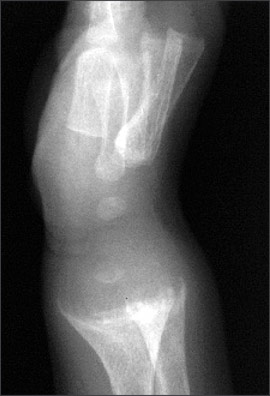
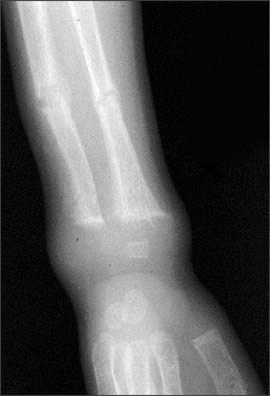
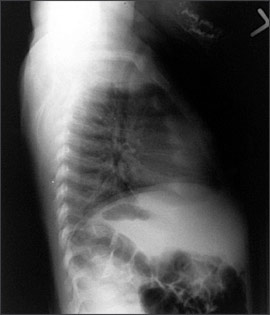
Treatment
Surgical intervention may be necessary to repair severe bony abnormalities, but for optimal healing to occur, the metabolic and nutritional imbalances have to be corrected first.31 Vitamin D and supplements of calcium and phosphorus are used to treat nutritional rickets.
Various vitamin D preparations, dosages (high versus low), dosing schedules (single versus multiple doses), and administration routes (oral or intramuscular) are available (Table 5).32,33 Ergocalciferol (Calciferol) is a useful medication for infants and children because it can be administered intramuscularly or orally in liquid or capsule form. The capsules can be softened in water and mixed with a palatable food such as applesauce.34 A single intramuscular or oral dose of various strengths (150,000 to 600,000 IU) of vitamin D in patients as young as three months has been studied and found to be adequate treatment for nutritional rickets.35–37 Hypercalcemia is more likely with oral doses greater than 300,000 IU.35
| Type of vitamin D | Trade names/available forms | Dosages | Mode of administration |
|---|---|---|---|
| Cholecalciferol (D3): 1 mg is equivalent to 40,000 IU | Vitamin D3, 1,000 IU tablets; Delta-D, 400 IU tablets | 125 to 250 mcg per day (5,000 to 10,000 IU) for two to three months or 15,000 mcg (600,000 IU) in four to six divided doses for one day | Oral |
| Ergocalciferol (D2) | Calciferol, Drisdol drops: liquid, 8,000 IU per mL; Drisdol capsules, 50,000 IU; Calciferol injection, 500,000 IU per mL | 25 to 125 mcg per day (1,000 to 5,000 IU) for six to 12 weeks33 | Oral or intramuscular |
| Dihydrotachysterol (DHT)*: 1 mg is approximately equivalent to 3 mg (120,000 IU) of vitamin D2 | DHT tablets, 0.125 mg, 0.2 mg, 0.4 mg; Intensol solution, 0.2 mg per mL; Hytakerol capsules, 0.125 mg | 0.5 mg as a single dose or 13 to 50 mcg per day until healed; 400 mcg of DHT is roughly equivalent to 1,250 mcg of ergocalciferol | Oral |
Researchers comparing a single intramuscular dose (600,000 IU) of vitamin D to a lower daily oral dosage (2,000 IU) for four weeks found that patients who received the intramuscular dose responded promptly without hypervitaminosis, whereas 40 percent of infants who received the oral dosages had no or minimal response.38 The physician must determine the best treatment strategy for each patient on a case-by-case basis. For example, if compliance is a major concern, the single intramuscular dose may be more appropriate.
After treatment initiation, all patients will require careful monitoring of serum calcium, phosphorus, alkaline phosphatase, and calcidiol levels and of urine calcium and phosphorus levels. A spot urine calcium to creatinine ratio should be followed to detect hypercalciuria. Adjustments to medications are made to accommodate any abnormal fluctuations in serum or urine values. The earliest biochemical change after treatment initiation is a rise in the level of phosphorus followed by calcium within the first week. Radiographic changes may be evident within a week, and physical examination findings may normalize within six months. No matter which treatment course is chosen, the physician has to closely monitor the child’s progress.
With regard to nutritional rickets, the most important role of the primary care physician is helping parents prevent it. Along with sun protection advice, measures needed to prevent nutritional rickets must be stressed to the child’s caregivers. Besides all exclusively breastfed infants, some older children also may need vitamin D supplementation. Parents should be encouraged to give their children foods that are high in calcium (Table 633 ).
| Food (approximate serving) | Amount of calcium (approximate mg) | |
|---|---|---|
| Breast milk (16 oz) | 125 | |
| Formula, cow’s milk–based (16 oz) | 265 | |
| Dairy products | ||
| Cheddar cheese (1 oz) | 200 | |
| Cow’s milk (1 cup) | 250 | |
| Ice cream (1 cup) | 150 | |
| Yogurt (4 oz) | 150 | |
| Fast foods | ||
| Cheeseburger | 20 | |
| Chicken nuggets (four to six pieces) | 13 | |
| French fries (small order) | 10 | |
| Pizza (one slice) | 145 | |
| Greens | ||
| Collard greens (1/2 cup, cooked) | 150 | |
| Spinach (1 cup, cooked) | 150 | |
Because vitamin D–dependent rickets, type I is caused by lack of production of calcitriol, treatment requires the replacement of that active product. The treatment of type II is more complex,39 and consultation with a children’s nephrologist is advised.
Familial hypophosphatemic rickets is treated with oral phosphorus and calcitriol (Rocaltrol), whereas hereditary hypophosphatemic rickets with hypercalciuria requires replacement of oral phosphorus alone. Investigators stress that treatment begun early in life lessens the disease burden.40 To ensure early treatment, infants of affected parents must be screened often for hypophosphatemia and increased levels of serum alkaline phosphatase.