
Am Fam Physician. 2007;75(4):501-506
A more recent article on common oral lesions is available.
This is part I of a two-part article on oral lesions. Part II, “Masses and Neoplasia,” appears in this issue of AFP .
Patient information: See related handout on canker sores.
Author disclosure: Nothing to disclose.
Common superficial oral lesions include candidiasis, recurrent herpes labialis, recurrent aphthous stomatitis, erythema migrans, hairy tongue, and lichen planus. Recognition and diagnosis require taking a thorough history and performing a complete oral examination. Knowledge of clinical characteristics such as size, location, surface morphology, color, pain, and duration is helpful in establishing a diagnosis. Oral candidiasis may present as pseudomembranous candidiasis, glossitis, or perlèche (angular cheilitis). Oral candidiasis is common in infants, but in adults it may signify immune deficiency or other illness. Herpes labialis typically is a mild, self-limited condition. Recurrent aphthous stomatitis most often is a mild condition; however, severe cases may be caused by nutritional deficiencies, autoimmune disorders, or human immunodeficiency virus infection. Erythema migrans is a waxing and waning disorder of unknown etiology. Hairy tongue represents elongation and hypertrophy of the filiform papillae and most often occurs in persons who smoke heavily. Oral lichen planus is a chronic inflammatory condition that may be reticular or erosive. Certain risk factors have been associated with each of these lesions, such as poor oral hygiene, age, tobacco use, and alcohol consumption, and some systemic conditions may have oral manifestations. Many recommended therapies for oral lesions are unsupported by randomized controlled trials.
The Surgeon General's report on oral health highlights the relationship between oral and overall health, emphasizing that oral health involves more than dentition.1 Physicians regularly encounter oral health issues in practice. For recognition and diagnosis of common oral lesions, a thorough history and a complete oral examination are required; knowledge of clinical characteristics such as size, location, surface morphology, color, pain, and duration also is helpful.
Large-scale, population-based screening studies have identified the most common oral lesions as candidiasis, recurrent herpes labialis, recurrent aphthous stomatitis, mucocele, fibroma, mandibular and palatal tori, pyogenic granuloma, erythema migrans, hairy tongue, lichen planus, and leukoplakia.2,3 This article, part I of a two-part series, reviews superficial mucosal lesions: candidiasis, herpes labialis, aphthous stomatitis, erythema migrans, hairy tongue, and lichen planus (Table 1).4–22 Part II covers masses and neoplasia.23
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| When treating recurrent herpes labialis with systemic antivirals such as acyclovir (Zovirax) or valacyclovir (Valtrex), therapy should be initiated during the prodrome. Topical penciclovir (Denavir) may help speed healing and reduce pain even if started after the prodrome. | B | 10, 12–14 |
| Patients with severe recurrent aphthous stomatitis should be evaluated for possible underlying systemic diseases and vitamin deficiencies. | B | 16, 34 |
| Condition | Clinical presentation | Treatment | Comments |
|---|---|---|---|
| Candidiasis4–9 | Pseudomembranous: adherent white plaques that may be wiped off | Topical antifungals (e.g., nystatin [Mycostatin] suspension or troches, clotrimazole [Mycelex] troches, fluconazole [Diflucan] suspension, or systemic antifungals (e.g., fluconazole, ketoconazole [Nizoral], itraconazole [Sporanox]) | Can confirm diagnosis with oral exfoliative cytology (stained with periodic acid-Schiff or potassium hydroxide), biopsy, or culture |
| Erythematous: red macular lesions, often with a burning sensation | |||
| Perlèche (angular cheilitis): erythematous, scaling fissures at the corners of the mouth | |||
| Recurrent herpes labialis10–14 | Prodrome (itching, burning, tingling) lasts approximately 12 to 36 hours, followed by eruption of clustered vesicles along the vermilion border that subsequently rupture, ulcerate, and crust | Immunocompetent patients usually do not require treatment | Reactivation triggers: ultraviolet light, trauma, fatigue, stress, menstruation |
| Topical agents include 1% penciclovir cream (Denavir) | |||
| Systemic agents (e.g., acyclovir [Zovirax], valacyclovir [Valtrex], famciclovir [Famvir]) are most effective if initiated during prodrome or as prophylaxis | |||
| Recurrent aphthous stomatitis15–17 | Ulcers surfaced by a yellowish-white pseudomembrane surrounded by erythematous halo | Mild cases do not require treatment | — |
| Fluocinonide gel (Lidex) or triamcinolone acetonide (Kenalog in Orabase), amlexanox paste (Aphthasol), chlorhexidine gluconate (Peridex) mouthwash | |||
| Erythema migrans18 | Migrating lesions with central erythema surrounded by white-to-yellow elevated borders; typically on tongue | Asymptomatic cases do not require treatment | — |
| Symptomatic cases may be treated with topical corticosteroids, zinc supplements, or topical anesthetic rinses | |||
| Hairy tongue19–21 | Elongated filiform papillae | Regular tongue brushing or scraping; avoidance of predisposing factors | Predisposing factors include smoking and poor oral hygiene as well as antibiotics and psychotropics |
| Lichen planus22 | Reticular: white, lacy striae | Asymptomatic cases do not require treatment | Buccal lesions typical in reticular form; other sites (e.g., tongue, gingiva) may be involved |
| Erosive: erythema and ulcers with peripheral radiating striae, erythematous and ulcerated gingiva | Symptomatic cases may be treated with a topical corticosteroid gel or mouth rinse |
Oral Candidiasis
As many as 60 percent of healthy adults carry Candida species as a component of their normal oral flora. However, certain local and systemic factors may favor overgrowth. These include use of dentures, use of a steroid inhaler, xerostomia, endocrine disorders, human immunodeficiency virus (HIV) infection, leukemia, malnutrition, reduced immunity based on age, radiation therapy, systemic chemotherapy, and use of broad-spectrum antibiotics or corticosteroids.4–6,24,25
Oral candidiasis typically is a localized infection; however, rarely it may progress to or occur in patients with systemic candidiasis. Clinical patterns of oral candidiasis are variable and include pseudomembranous candidiasis, or thrush (Figure 1); median rhomboid glossitis and other forms of erythematous candidiasis (Figure 2); and perlèche, or angular cheilitis (Figure 3). Risk factors for systemic infection include acquired immunodeficiency syndrome, diabetes, hospitalization, immunosuppressive therapy, malignancy, neutropenia, organ transplantation, and prematurity.26,27
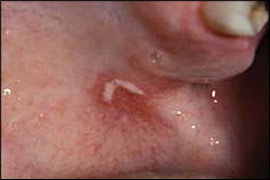
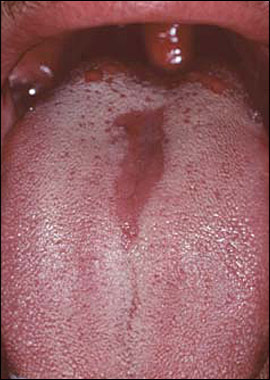
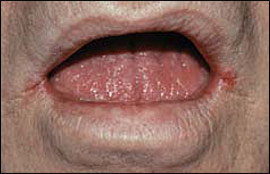
Oral candidiasis is common in infants, affecting 1 to 37 percent of newborns.28 Candidiasis in otherwise healthy infants manifests as a minor infection of the oral cavity, oropharynx, or skin (e.g., candidal diaper dermatitis).29 In contrast, candidiasis among preterm or hospitalized critically ill infants can, in rare instances, become systemic and potentially fatal.27 Chronic mucocutaneous candidiasis in infancy or early childhood can be associated with the development of autoimmune endocrine disorders, such as hypoparathyroidism, hypoadrenalism, hypothyroidism, and diabetes mellitus.5
Treatment involves topical or systemic antifungals. Commonly used topical regimens include nystatin (Mycostatin; not absorbed), clotrimazole (Mycelex troche), and systemic fluconazole (Diflucan). Randomized controlled trials have demonstrated fluconazole suspension to be more effective than nystatin in normal and immunocompromised children.7 Systemic agents such as fluconazole, ketoconazole (Nizoral), and itraconazole (Sporanox) may be used for patients who have candidiasis refractory to topical therapy, are intolerant of topical agents, or are at high risk of developing systemic infection.8,9
Herpes Labialis
Primary oral infection with the herpes simplex virus (HSV) typically occurs at a young age, is asymptomatic, and is not associated with significant morbidity. A minority of persons develop a symptomatic primary infection, presenting with an acute outbreak of oral vesicles that rapidly collapse to form zones of erythema and ulceration. In all cases, the gingiva is involved; in addition, other oral mucosal sites and the perioral skin may be affected. Concomitant cervical lymphadenopathy, fever, chills, anorexia, and irritability are common findings.
After primary oral infection, HSV may persist in a latent state in the trigeminal ganglion and later reactivate as the more common herpes labialis, or “cold sores.” Common triggers for reactivation are well known and include ultraviolet light, trauma, fatigue, stress, and menstruation. These lesions affect approximately 15 to 45 percent of the U.S. population.10 They classically manifest as a well-localized cluster of small vesicles along the vermilion border of the lip or adjacent skin (Figure 4). The vesicles subsequently rupture, ulcerate, and crust within 24 to 48 hours. Spontaneous healing occurs over seven to 10 days.
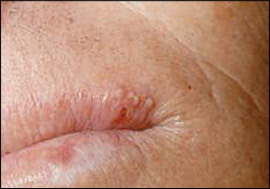
In immunocompetent patients, herpes labialis usually is mild and self-limited. However, pain, swelling, and cosmetic concerns may prompt physician consultation. Orally administered antiviral agents, such as acyclovir (Zovirax) or valacyclovir (Valtrex), have a modest clinical benefit if initiated during the prodrome.10,11,30 Topical treatment with 1% penciclovir cream (Denavir) may reduce healing time and pain slightly, even if initiated after the prodrome.12,30 Reduction in healing time with systemic or topical agents is modest—approximately one day or less. Use of systemic antivirals for herpes labialis generally should be reserved for immunocompromised patients. Prophylactic treatment with oral antiviral medications may help patients who experience frequent recurrences, anticipate unavoidable exposure to a known trigger, or suffer from frequent episodes of postherpetic erythema multiforme.13,14,31,32 Recurrent herpetic infections should not be treated with corticosteroids.
Recurrent Aphthous Stomatitis
Recurrent aphthous stomatitis, or “canker sores,” is an oral ulcerative condition with a prevalence ranging from 5 to 21 percent.33 Although a variety of host and environmental factors have been implicated, the precise pathogenesis remains unknown. Smoking is associated with a lower prevalence, but other associations, such as nutritional deficiencies (e.g., vitamin B12, folate, iron), remain unclear.33 Severe cases may be related to underlying systemic conditions such as inflammatory bowel disease, celiac disease, Behçet's syndrome, and HIV infection.34,35
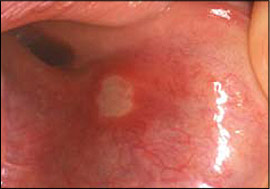
Recurrent aphthous stomatitis is characterized by recurring, painful, solitary or multiple ulcers, typically covered by a white-to-yellow pseudomembrane and surrounded by an erythematous halo (Figure 5). Recurrent aphthous stomatitis usually involves nonkeratinizing mucosa (e.g., labial mucosa, buccal mucosa, ventral tongue). There are three clinical forms: minor, major, and herpetiform. The minor form is the most common and appears as rounded, well-demarcated, single or multiple ulcers less than 1 cm in diameter that usually heal in 10 to 14 days without scarring.
Most patients with mild aphthae require no treatment or only periodic topical therapy. Commonly used therapies include topical corticosteroids, such as fluocinonide gel (Lidex) and triamcinolone acetonide with carboxymethylcellulose paste (Kenalog in Orabase). However, much of the evidence in support of these treatments is from small, incompletely blinded trials, and thus their effectiveness is uncertain.36,37 Chlorhexidine gluconate (Peridex) mouthwash decreases the severity of an episode but does not reduce the incidence of ulcers.15 Amlexanox 5% paste (Aphthasol) may promote healing and lessen pain.16 In severe or constantly recurring cases, systemic therapy with agents such as thalidomide (Thalomid) may be necessary. Because of the risk of serious adverse effects and its off-label status, thalidomide generally is reserved for severe cases such as those associated with HIV infection.17
Erythema Migrans
Erythema migrans, which should not be confused with the characteristic rash of early Lyme disease, also is known as geographic tongue or benign migratory glossitis. A common oral inflammatory condition of unknown etiology, it has an estimated prevalence of 1 to 3 percent. The most commonly suggested associations are atopy and psoriasis.18 It usually affects the tongue, although other oral sites may be involved.
Erythema migrans may occur in children and adults and exhibits a female predilection. Tongue lesions exhibit central erythema caused by atrophy of the filiform papillae and usually are surrounded by slightly elevated, curving, white-to-yellow borders (Figure 6). The condition typically waxes and wanes, and the lesions demonstrate a migrating pattern. Some patients may complain of pain or burning, especially when eating spicy foods. However, most individuals are asymptomatic and do not require treatment for this benign condition. For symptomatic cases, several treatments have been proposed, including topical steroids, zinc supplements, and topical anesthetic rinses. None of these treatments has been proven to be uniformly effective.18
Hairy Tongue
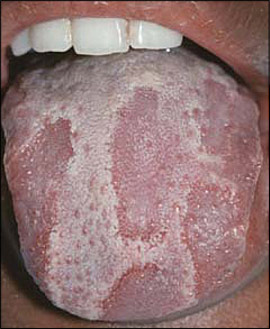
Hairy tongue is characterized by elongation and hypertrophy of the filiform papillae on the dorsal tongue, causing a hair-like appearance (Figure 7). This condition results from inadequate desquamation or increased keratinization of the papillae. These papillae, which normally are about 1 mm in length, may become as long as 12 mm. It occurs most often in persons who smoke heavily and it also may be associated with poor oral hygiene, oxidizing mouthwashes, Candida albicans, and certain medications.19–21
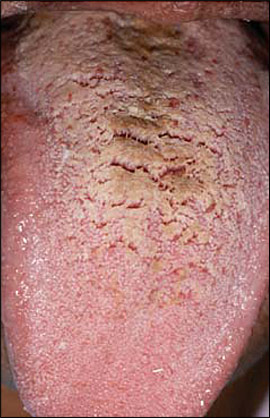
Although often called “black” hairy tongue, the condition may cause black, brown, or yellow discoloration depending on the foods ingested, tobacco use, and the amount of coffee or tea consumed. Rarely, patients may complain of gagging or of a metallic taste. Debris between elongated papillae can result in halitosis. Most cases improve with avoidance of predisposing factors and regular tongue brushing using a soft toothbrush or tongue scraper. Hairy tongue should not be confused with oral hairy leukoplakia, a condition characterized by vertical white striations typically affecting the lateral tongue bilaterally.
Lichen Planus
Oral lichen planus is a chronic waxing and waning inflammatory condition that affects an estimated 1 to 2 percent of adults. Although the etiology is uncertain, evidence suggests an immune-mediated mechanism involving CD8+ cytotoxic T-cell–induced apoptosis of epithelial cells.38 All age groups may be affected, but it predominates in adults older than 40 years, with a female-to-male ratio of 1.4:1.39
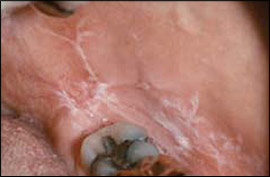
Two major clinical forms of oral lichen planus exist: reticular and erosive. The reticular form can appear as bilateral asymptomatic, white, lacy striations (Wickham's striae) or papules on the posterior buccal mucosa (Figure 8). The erosive form manifests as zones of tender erythema and painful ulcers surrounded by peripheral white, radiating striae (Figure 9A). It may also manifest as generalized erythema and ulceration of the gingiva, known as desquamative gingivitis (Figure 9B).
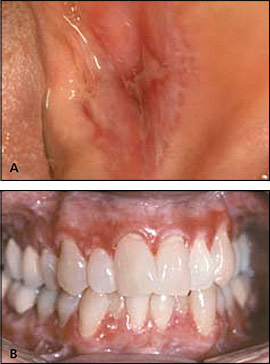
Classic lesions of reticular form often are readily identified clinically. However, lesions that do not exhibit classic features may require biopsy for diagnosis. Asymptomatic patients do not require treatment. For symptomatic patients, topical corticosteroid gels, such as fluocinonide and corticosteroid mouth rinses, may be prescribed.22 There is debate about whether oral lichen planus is associated with an increased risk of oral cancer.40 Therefore, periodic follow-up of patients is appropriate.