
Am Fam Physician. 2007;75(5):683-688
Patient information: See related handout on asbestosis, written by the authors of this article.
Author disclosure: William S. Beckett, M.D., is supported by NIEHS P30 ES01247 and the New York State Occupational Health Clinics Network. Patricia J. Sime, M.D., is supported by NIH RO1HL075432, NIH K08HL04492, and NIEHS P30 ES01247.
The inhalation of asbestos fibers may lead to a number of respiratory diseases, including lung cancer, asbestosis, pleural plaques, benign pleural effusion, and malignant mesothelioma. Although exposure is now regulated, patients continue to present with these diseases because of the long latent period between exposure and clinical disease. Presenting signs and symptoms tend to be nonspecific; thus, the occupational history helps guide clinical suspicion. High-risk populations include persons in construction trades, boilermakers, shipyard workers, railroad workers, and U.S. Navy veterans. Every effort should be made to minimize ongoing exposure. Patients with a history of significant asbestos exposure may warrant diagnostic testing and follow-up assessment, although it is unclear whether this improves outcomes. Patients with significant exposure and dyspnea should have chest radiography and spirometry. The prognosis depends on the specific disease entity. Asbestosis generally progresses slowly, whereas malignant mesothelioma has an extremely poor prognosis. The treatment of patients with asbestos exposure and lung cancer is identical to that of any patient with lung cancer. Because exposure to cigarette smoke increases the risk of developing lung cancer in patients with a history of asbestos exposure, smoking cessation is essential. Patients with asbestosis or lung cancer should receive influenza and pneumococcal vaccinations.
Asbestos, a crystalline mineral that occurs in deposits throughout the world, is the smallest naturally occurring fiber. Because of its flexibility, durability, and resistance to heat and chemical corrosion, it became widely used in industry. The inhalation of asbestos fibers was first linked to the development of lung disease in 1890, and the first deaths attributable to asbestos exposure were reported in 1907. Legislation controlling exposure was introduced in the United Kingdom in 1931, but the United States did not enact its first legislation limiting exposure until 1971.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Risk of asbestos exposure should be assessed with occupational history. Screening should be considered in patients with a high risk of exposure. | C | 2 |
| Chest radiography and pulmonary function testing should be performed every three to five years in patients with asbestos-related disease. | C | 7 |
| Smoking cessation should be encouraged. | A | 3, 9, 12 |
| Influenza and pneumococcal vaccines should be given to patients with asbestosis or cancer. | C | 12 |
The peak global incidence of asbestos-related disease is expected to occur 30 to 40 years after the period of peak usage (i.e., the 1960s and 1970s). For diffuse malignant mesothelioma, the condition with the longest latency, the incidence is expected to rise in Europe until 2020.1 The prevalence of asbestosis in the United States is not known, but in 2000, there were an estimated 20,000 hospital discharges with this diagnosis and 2,000 deaths with asbestosis as the underlying or contributing cause, and these numbers are expected to rise through this decade.2,3 The incidence of malignant mesothelioma in the United States was thought to peak at 2,000 cases per year from 2000 to 2004; another study suggested there would be a yearly average of 3,200 deaths from asbestos-related lung cancer from 1985 to 2009.4,5
Asbestos-Related Lung Disease
Inhalation of asbestos fibers can result in a number of distinct pathologic processes (Table 1).2–5 These include the development of pleural-based abnormalities such as benign plaques, benign pleural effusions, and malignant mesothelioma. In addition, new cases of asbestosis, an important pneumoconiosis, and many asbestos-related lung cancers continue to occur. Most patients with asbestos-related lung disease have a strong exposure history; however, significant disease can occur with minimal exposure and, rarely, with unknown exposure.
| Disease | Presenting symptoms | Prevalence* | Treatment |
|---|---|---|---|
| Asbestosis | Dyspnea, dry cough | Approximately 200,000 patients with asbestosis and 2,000 deaths annually | No specific therapy; general measures outlined in the article; surveillance for lung cancer; smoking cessation |
| Lung cancer | Chest pain, cough, dyspnea, hemoptysis, weight loss, fatigue, symptoms caused by metastases and direct invasion | Estimated 2,000 to 3,200 lung cancer deaths annually related to asbestos exposure | Multimodality treatment including surgery, radiotherapy, and chemotherapy |
| Mesothelioma | Chest pain, cough, dyspnea, weight loss, fatigue, pleural effusion, symptoms caused by metastases, pericardial invasion, esophageal compression, superior vena caval invasion | Approximately 2,000 deaths annually; incidence and mortality rate are the same | Multidisciplinary approach focused on supportive care; multimodality treatment including surgery, radiotherapy, and chemotherapy (clinical trials ongoing); radiotherapy for localized pain and metastatic spread occurring along a biopsy tract; chemical or surgical pleurodesis for pleural effusions |
| Pleural plaques | Usually asymptomatic, incidental finding on chest radiography; may cause grating sensation associated with calcified plaques | Among exposed persons: 3 to 58 percent; general population: between 0.5 and 8 percent | Smoking cessation; withdrawal from further exposure; management of concurrent and other respiratory diseases |
Although occupational exposure is now regulated in the United States, past exposure must be recognized. It is important to take a comprehensive occupational and environmental history to identify persons at risk because of previous exposure. Factors to establish include the specific occupation (Table 2), the duration of that occupation, and the intensity of exposure (e.g., was the dust visible or not). A significant exposure can be defined as at least several months' exposure to visible dust that began more than 10 years earlier. Although much attention is focused on industrial exposure, environmental sources also play a role. These include residence near asbestos or vermiculite mines and prolonged exposure in buildings with open sources of contamination.6 Undisturbed insulation in good condition is not hazardous.7
| Asbestos-containing products |
| Asbestos-containing flight materials: exposed workers may include aircraft mechanics and those involved in aerospace and missile production and aircraft manufacturing |
| Asbestos-lined electrical products: exposed workers may include electrical workers (e.g., electricians), electrical linemen, telephone linemen, and power plant workers |
| Asbestos shipping materials: exposed workers may include product shipyard workers (e.g., insulators, laggers, painters, pipe fitters, maintenance workers, welders), Coast Guard personnel, merchant mariners, longshoremen, U.S. Navy personnel, asbestos manufacturing plant workers, insulators, machinists, persons working at packing and gasket manufacturing plants, pipe fitters, and power plant workers |
| Brake linings and clutch pads: exposed workers may include auto mechanics, those involved in brake and clutch manufacturing, and assembly workers |
| Building materials: exposed workers may include building engineers, cement plant production workers, building material manufacturers, construction workers (including insulators, boilermakers, steelworkers, ironworkers, plumbers, steamfitters, plasterers, drywallers, cement and masonry workers, roofers, tile/linoleum installers, carpenters, and welders) |
| Other asbestos-containing products: exposed workers may include railroad workers, steamfitters, refinery workers, sheet metal workers, refractory products plant workers, rubber workers, and warehouse workers |
| Asbestos removal |
| Removal of insulation, asbestos removal, and waste handling |
| Building demolition and ship breaking |
| Environmental exposure |
| Asbestos in public buildings (e.g., hospitals, libraries, schools); occurs when the asbestos is disturbed during building or maintenance work |
| Family members of persons exposed occupationally |
| Asbestos production |
| Asbestos mining; textile mill workers who weave asbestos into cloth |
| Asbestos transport |
| Packing and handling of asbestos |
Screening for asbestos-related disease may be appropriate in persons with a history of significant asbestos exposure; however, screening of the general population is not warranted. If a person with a history of significant exposure reports exertional dyspnea, the initial assessment should include spirometry and chest radiography in addition to a history and physical examination. If there is no evidence of abnormalities, high-resolution computed tomography (CT) may be considered because this may reveal pleural-based plaques and is more sensitive than chest radiography at detecting these lesions and mild fibrosis. The presence of plaques indicates significant asbestos exposure. Full pulmonary function tests, including measurement of lung volumes and diffusion capacity, should be performed in patients with abnormal spirometry results, imaging abnormalities, or suspected asbestos-related conditions.7 Symptomatic patients may be entitled to workers' compensation if there is loss of employment or functional impairment.
The presence of asbestosis is an independent risk factor for the development of lung cancer. Thus, the appearance of symptoms such as dyspnea, cough, chest discomfort, or weight loss necessitates a prompt and full assessment. Current recommendations support lifelong surveillance for persons with a significant exposure history or ongoing exposure. The American Thoracic Society recommends performing chest radiography and pulmonary function testing every three to five years in patients with disease.7 There is no good evidence that screening improves outcomes, although it may help identify lung cancer earlier. Screening for mesothelioma is not helpful.
LUNG CANCER
Asbestos exposure significantly increases the risk of developing small cell and non–small cell lung carcinoma.8 A number of studies suggest an increased incidence of non–small cell lung carcinoma in patients with asbestosis compared with those who have been exposed to asbestos but do not have asbestosis.8 Lung cancer can occur in nonsmokers exposed to asbestos; however, the risk is magnified several-fold by smoking.9–11 All patients who smoke cigarettes must be warned about this risk, and every attempt should be made to assist patients with smoking cessation. Nonmesothelioma asbestos-related lung cancers are indistinguishable clinically from lung cancers related to smoking alone. Thus, evaluation of a new noncalcified pulmonary nodule is similar in patients with or without a history of asbestos exposure. Patients with lung cancer should receive influenza and pneumococcal vaccines.12
ASBESTOSIS
Asbestosis is a fibrotic lung disease, or pneumoconiosis, resulting from the inhalation of asbestos fibers. In many patients, it is characterized by a very mild and indolent fibrosis that usually produces relatively minor symptoms. In general, the latent period between the peak asbestos exposure and diagnosis is 20 to 30 years.
Complaints of exertional dyspnea associated with auscultatory crackles on physical examination should prompt further investigation. The first changes in pulmonary function may be decreased diffusion capacity and exertional oxygen desaturation. As the process becomes more advanced, pulmonary function tests will reveal a restrictive pattern with a decreased total lung capacity and vital capacity.
Chest radiography typically demonstrates increased interstitial markings, usually more prominent in the lower lobes, and often pleural plaques. Typical findings on high-resolution CT of the chest include increased interstitial markings, predominantly at the bases; later, honeycombing may be apparent (Figure 1). In many respects, asbestosis is clinically similar to idiopathic pulmonary fibrosis, but asbestosis usually progresses slowly, whereas idiopathic pulmonary fibrosis has a rapidly progressive course. No current treatment effectively alters the natural course of asbestosis. Patients will benefit from influenza and pneumococcal vaccines.12
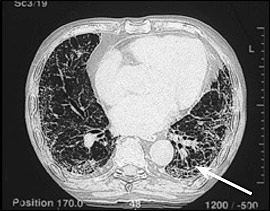
Given a history of significant occupational asbestos exposure and typical high-resolution CT findings, surgical lung biopsy rarely is needed to establish a diagnosis.13 For patients in whom surgical lung biopsy is performed, the pathologic pattern is that of usual interstitial pneumonia. This is the same pathology occurring in patients with idiopathic pulmonary fibrosis and may also be seen in pulmonary fibrosis associated with collagen vascular diseases (e.g., rheumatoid arthritis).13 Asbestos bodies are identified by special iron staining of tissue, and the number of these bodies correlates with the severity of fibrosis. Their presence in the lung tissue confirms the diagnosis of asbestosis.
BENIGN PLEURAL DISEASE
The most common pathologic pulmonary response to asbestos inhalation is the development of pleural plaques (Figure 2). Over time, collagen is deposited in the pleura and may calcify. Most plaques are completely asymptomatic, and there is no evidence that plaques transform into malignant lesions. Plaques occur in approximately 50 percent of persons with heavy and prolonged exposure to asbestos and are, therefore, a marker of asbestos exposure.7 Plaques are not always visible on plain chest radiography, but high-resolution CT will identify up to 50 percent of plaques found at autopsy. However, chest radiography usually is adequate, and the use of high-resolution CT is reserved most often for diagnostic uncertainty or confirmatory testing.7
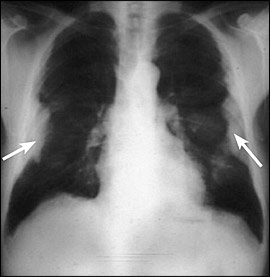
Benign asbestos pleural effusions, usually unilateral, are the most common manifestation of asbestos-related pleural disease within 10 to 20 years after exposure.14 When followed over time, effusions may wax and wane. The development of any new pleural effusion mandates a thorough evaluation, including tuberculosis skin testing and diagnostic thoracentesis. Asbestos pleural effusions are exudative.7 However, in cases of exudative pleural effusions, a pleural biopsy may be needed to evaluate for tuberculosis and malignancy. Furthermore, pleural effusion with pleuritic pain may be a manifestation of malignant mesothelioma.15 Therefore, benign asbestos pleural effusion is a diagnosis of exclusion.
DIFFUSE MALIGNANT MESOTHELIOMA
Diffuse malignant mesothelioma is an aggressive tumor derived from mesothelial cells, most commonly of the pleura. It is uniformly fatal, with a median survival time of six to 18 months from diagnosis. Among persons who have worked with asbestos, the lifetime risk of developing mesothelioma is high, although the condition is relatively uncommon, with approximately 2,000 new cases per year in the United States.4 However, even relatively low-level exposure has been associated with an increased risk of developing mesothelioma.13
The presenting symptoms of malignant mesothelioma are vague (Table 1), which often leads to a delay before the patient seeks care. Similarly, the nonspecific nature of the symptoms makes the diagnosis difficult. Chest pain and dyspnea are common initial complaints.16 Chest radiography most often will reveal a large, unilateral pleural effusion. Chest CT will demonstrate the same features; however, irregular thickening of the pleura also may be visible. In more advanced disease, there may be superior vena cava syndrome, Horner's syndrome, dysphagia, or other complications resulting from the propensity of mesothelioma to invade neighboring structures. Pathologic diagnosis can prove difficult, and many cases are misdiagnosed initially.
Palliative radiation therapy can be effective in reducing symptoms, especially from metastases. Current clinical trials emphasize a combination of surgery, radiation, and chemotherapy, but no regimen has yet been clearly shown to improve survival rates. Recent study has focused on the identification of serum markers (e.g., serum mesothelin-related protein, osteopontin) that may prove useful as screening tools.17,18