
Am Fam Physician. 2007;75(8):1248-1250
Background: Irritable bowel syndrome (IBS) is a chronic disorder associated with altered bowel habits and abdominal pain and discomfort. To date, there is no accepted comprehensive pathologic explanation for IBS. Pimentel and colleagues conducted a randomized controlled trial to investigate whether administration of a nonabsorbed oral antibiotic (rifaximin [Xifaxan]) improves symptoms in patients with IBS.
The Study: The study population included 87 patients between 18 and 65 years of age. Eligible patients met Rome I criteria, a classification system for functional gastrointestinal disorders. Patients were excluded if they had underlying conditions known to produce overgrowth of intestinal bacterial flora, had taken oral antibiotics in the previous three months, or were currently taking tegaserod (Zelnorm) or antidepressants. Before beginning the study, patients completed a seven-day stool diary and a questionnaire assessing their symptoms during that period. Next, participants received a 10-day course of 400 mg rifaximin or placebo three times a day.
After completing the 10-day course, patients again recorded a seven-day stool diary and a follow-up questionnaire. In the follow-up phase of the study, patients completed questionnaires weekly for nine weeks. The questionnaires used throughout the study assessed the severity of the patients' IBS symptoms. The symptoms chosen for treatment end point assessment were diarrhea, constipation, abdominal pain, and bloating. The patients reported their global improvement in overall IBS symptoms on a scale from 0 to 100 percent.
Results: Comparison of the global improvement scores of rifaximin versus placebo groups across the 10-week follow-up demonstrated an average increase of 36.4 versus 21.0 percent, respectively (see accompanying figure). Patient reports of symptom severity demonstrated a significant improvement in bloating but no significant differences in abdominal pain, diarrhea, or constipation. The most common adverse effects were abdominal pain, diarrhea, and a bad taste in the mouth; however, these were rare and the incidences reported were similar in both groups.
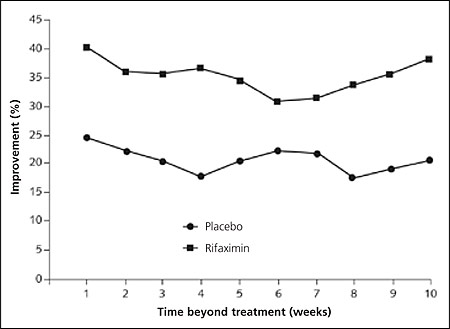
Conclusion: The authors conclude that the use of the antibiotic rifaximin resulted in a greater global improvement in IBS than placebo. The authors noted that improvements continued through 10 weeks of follow-up despite cessation of antibiotic treatment after 10 days. They feel that this new concept of IBS treatment warrants future studies that allow direct comparison of antibiotic treatment with other treatment strategies such as prokinetics and probiotics.