
A more recent article on treatment of chronic insomnia is available.
Am Fam Physician. 2007;76(4):517-526
Patient information: See related handout on insomnia, written by the authors of this article.
Author disclosure: Nothing to disclose.
The frequency of sleep disruption and the degree to which insomnia significantly affects daytime function determine the need for evaluation and treatment. Physicians may initiate treatment of insomnia at an initial visit; for patients with a clear acute stressor such as grief, no further evaluation may be indicated. However, if insomnia is severe or long-lasting, a thorough evaluation to uncover coexisting medical, neurologic, or psychiatric illness is warranted. Treatment should begin with nonpharmacologic therapy, addressing sleep hygiene issues and exercise. There is good evidence supporting the effectiveness of cognitive behavior therapy. Exercise improves sleep as effectively as benzodiazepines in some studies and, given its other health benefits, is recommended for patients with insomnia. Hypnotics generally should be prescribed for short periods only, with the frequency and duration of use customized to each patient's circumstances. Routine use of over-the-counter drugs containing antihistamines should be discouraged. Alcohol has the potential for abuse and should not be used as a sleep aid. Opiates are valuable in pain-associated insomnia. Benzodiazepines are most useful for short-term treatment; however, long-term use may lead to adverse effects and withdrawal phenomena. The better safety profile of the newer-generation non-benzodiazepines (i.e., zolpidem, zaleplon, eszopiclone, and ramelteon) makes them better first-line choices for long-term treatment of chronic insomnia.
The American Academy of Sleep Medicine defines insomnia as unsatisfactory sleep that impacts daytime functioning.1 More than one third of adults report some degree of insomnia within any given year, and 2 to 6 percent use medications to aid sleep.2 Insomnia is associated with increased morbidity and mortality caused by cardiovascular disease and psychiatric disorders and has other major public health and social consequences, such as accidents and absenteeism.3 Risk factors for chronic insomnia include increasing age, female sex, psychiatric illness, medical comorbidities, impaired social relationships, lower socioeconomic status, separation from a spouse or partner, and unemployment.4
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Exercise, cognitive behavior therapy, and relaxation therapy are recommended as effective, nonpharmacologic treatments for chronic insomnia. | A | 4, 7, 12, 16 |
| Melatonin is effective in patients with circadian rhythm sleep disorders and is safe when used in the short term. | B | 20 |
| Benzodiazepines are effective for treating chronic insomnia but have significant adverse effects and the risk of dependency. | B | 4, 22, 36 |
| Nonbenzodiazepines (e.g., eszopiclone [Lunesta], zaleplon [Sonata], zolpidem [Ambien]) are effective treatments for chronic insomnia and, based on indirect comparisons, appear to have fewer adverse effects than benzodiazepines. | B | 4 |
Evaluation
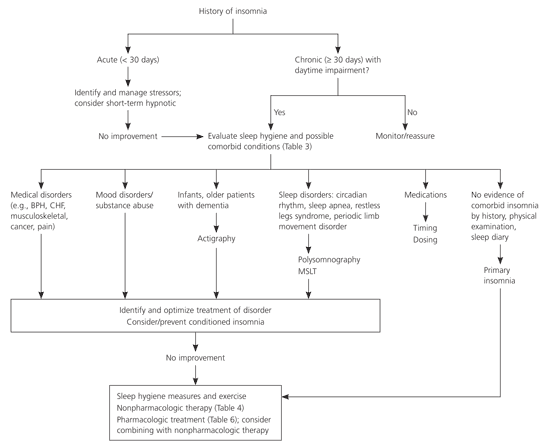
Criteria for the diagnosis of insomnia are provided in Table 1.5 Although there are several classification systems, it is practical to divide insomnia into two categories by duration: acute (i.e., less than 30 days) and chronic (i.e., 30 days or longer). If insomnia is associated with another condition, it is designated as comorbid insomnia (Table 2).6–8 Only about 15 to 20 percent of patients with chronic insomnia have no other associated diagnosis (primary insomnia).9
| At least one of the following complaints: |
| Difficulty initiating and/or maintaining sleep; sleep that is poor in quality; trouble sleeping despite adequate opportunity and circumstances for sleep; waking up too early |
| At least one of the following types of daytime impairment related to sleep difficulty: |
| Attention, concentration, or memory impairment; concerns or worries about sleep; daytime sleepiness; errors or accidents at work or while driving; fatigue or malaise; gastrointestinal symptoms; lack of motivation; mood disturbance or irritability; social or vocational dysfunction or poor school performance; tension headaches |
| Selected causes of acute insomnia (< 30 days)* | |
| Situational stress (e.g., occupational, interpersonal, financial, academic, medical) | |
| Environmental stressors (e.g., noise) | |
| Death or illness of a loved one | |
| Selected causes of chronic insomnia (≥ 30 days) | |
| Medical disorders | |
| Arthropathies, cancer, chronic pain, congestive heart failure, COPD, end-stage renal disease, gastroesophageal reflux disease, HIV/AIDS, hyperthyroidism, nocturia caused by prostatic hypertrophy, stroke | |
| Medications | |
| Anticholinergic agents; antidepressants (SSRIs, bupropion [Wellbutrin]), MAOIs; antiepileptics (lamotrigine [Lamictal], phenytoin [Dilantin]); antineoplastics; beta blockers; bronchodilators (beta agonists); CNS stimulants (methylphenidate [Ritalin], dextroamphetamine [Dextrostat], nicotine [Nicotrol]); interferon alfa; miscellaneous (diuretics, atorvastatin [Lipitor], levodopa, quinidine); steroids, oral contraceptives, progesterone, thyroid hormone | |
| Primary sleep disorder | |
| Periodic limb movement disorder, restless legs syndrome, sleep apnea | |
| Psychiatric disorders | |
| Anxiety disorders, bipolar disorder or schizophrenia, major depressive or dysthymic disorders, personality disorders, post-traumatic stress disorder | |
| Sleep–wake schedule disorder | |
| Irregular sleep–wake cycle, jet lag, shift work | |
| Substance abuse | |
| Alcohol, caffeine, drug withdrawal, stimulants (e.g., amphetamines, methamphetamines) | |
The frequency of sleep disruption and the degree to which insomnia significantly affects daytime function (e.g., quality of life, work limitations, mood/social life) are probably the most important determinants of the need for evaluation and treatment. If the initial evaluation identifies an acute stressor such as grief or noise, no further evaluation is indicated and treatment can be initiated. A more comprehensive evaluation should be pursued with nonresponders or if a comorbid condition is present or suspected.
The evaluation of chronic insomnia should involve a detailed history and examination to detect any coexisting medical or psychiatric illness and may include an interview with a partner or caregiver. Evaluation should include an assessment of sleep dysfunction and a sleep diary (Table 3).3,8,10 Following this evaluation, the need for further testing or pharmacotherapy can be determined.3,8,10,11
| History and examination | |
| Helps detect any coexisting medical or psychiatric illness | |
| Sleep history must span the entire day and should include an interview with the partner or caregiver | |
| Interview partner or caregiver about patient's sleep habits, daytime functioning, substance use (e.g., alcohol, tobacco, caffeine), snoring, apnea, and unusual limb movement | |
| Take medication history; physical examination should include neurologic examination, Mini-Mental State Examination | |
| Sleep diary | |
| A two-week sleep diary should record information on bedtime, rising time, daytime naps, sleep-onset latency, number of nighttime awakenings, total sleep time, and the patient's mood on arousal | |
| Questions should include daytime symptoms such as somnolence and frequency of napping | |
| Polysomnography, multiple sleep latency testing | |
| Useful if sleep apnea or periodic limb movement disorder is suspected | |
| Use when behavioral and psychopharmacologic treatments are unsuccessful | |
| Actigraphy | |
| An activity monitor or motion detector, typically worn on the wrist, records movement; the absence of movement for a given continuous period is consistent with sleep | |
| Useful in evaluating sleep patterns in patients with insomnia, analyzing the beneficial effects of treatment measures, diagnosing circadian rhythm disorders, and evaluating sleep in patients unable to tolerate polysomnography | |
| Neuroimaging | |
| Use if a structural lesion (e.g., mass lesion, arteriovenous malformation) is suspected on history and examination |
Treatment Overview
Ideally, treatment for insomnia would improve sleep quantity and quality, improve daytime function (greater alertness and concentration), and cause minimal adverse drug effects. An approach to the evaluation and treatment of the patient with insomnia is shown in Figure 1. Most experts recommend starting with nonpharmacologic therapy (Table 4).4,7,12–17 Good evidence supports a benefit for relaxation therapy and cognitive behavior therapy (CBT)4,12 that may be sustained over six to 24 months.13–15 Exercise improves sleep as effectively as benzodiazepines in some studies and, given its other health benefits, is recommended for patients with insomnia.7,16 Behavioral and cognitive interventions have minimal risk of adverse effects, but disadvantages include high initial cost, lack of insurance coverage, few trained therapists, and decreased effectiveness in older adults.17
| Treatment | Description |
|---|---|
| Cognitive behavior therapy | Helps change incorrect beliefs and attitudes about sleep (e.g., unrealistic expectations, misconceptions, amplifying consequences of sleeplessness); techniques include reattribution training (i.e., goal setting and planning coping responses), decatastrophizing (aimed at balancing anxious automatic thoughts), reappraisal, and attention shifting |
| Exercise | Moderate-intensity exercise (should not occur just before bedtime) |
| Relaxation therapy | Tensing and relaxing different muscle groups; biofeedback or imagery (visual and auditory feedback) to reduce somatic arousal; meditation; hypnosis |
| Sleep restriction (paradoxical intention therapy) | Uses a paradoxical approach in which the patient spends less time in bed (by associating time spent in bed with time spent sleeping) |
| Bedtimes are then increased or decreased progressively depending on improvement or deterioration of sleep quality and duration | |
| This state of minimal sleep deprivation eventually leads to more efficient sleep | |
| Stimulus control therapy | Avoid bright lights (including television); noise and temperature extremes; and large meals, caffeine, tobacco, and alcohol at night |
| Minimize evening fluid intake; leave the bedroom if unable to fall asleep within 20 minutes; limit use of the bedroom to sleep and intimacy | |
| Temporal control measures | Consistent time of wakening; minimal daytime napping |
Pharmacologic Therapy
Hypnotics are recommended when immediate symptom response is desired, when insomnia produces serious impairment, when nonpharmacologic measures do not produce the desired improvement, or when insomnia persists after treatment of an underlying medical condition. Table 5 outlines prescribing guidelines for hypnotics.18
| Initiate hypnotic use with identifying and addressing specific behaviors, circumstances, and underlying disorders contributing to insomnia |
| Prescribe the lowest effective dose of the hypnotic |
| Prescribe hypnotics for short durations (two to four weeks) and intermittently (duration based on patient's return to an acceptable sleep cycle) |
| Avoid hypnotic use or exercise caution if patient has a history of substance abuse, myasthenia gravis, respiratory impairment, or acute cerebrovascular accident |
| Watch for requests for escalating doses or resistance to tapering or discontinuing hypnotic |
| Hypnotics should be discontinued gradually (i.e., tapered); physician should be alert for adverse effects (especially rebound insomnia) and withdrawal phenomena |
ANTIHISTAMINES
Nearly 25 percent of patients with insomnia use over-the-counter (OTC) sleep aids, and 5 percent use them at least several nights a week. Routine use of OTC antihistamines such as diphenhydramine (Benadryl) and doxylamine (Unisom) should be discouraged because they are only minimally effective in inducing sleep, may reduce sleep quality, and can cause residual drowsiness.3,8
HERBAL AND DIETARY SUPPLEMENTS
Many herbs and dietary supplements (e.g., valerian root, melatonin, lavender, passionflower, kava, St. John's wort, glutamine, niacin, and l-tryptophan) have been promoted as sleep aids.19 There is insufficient evidence of benefit except for melatonin and valerian.
Melatonin, a hormone produced by the pineal gland that is involved in sleep regulation, improves insomnia caused by circadian schedule changes (e.g., jet lag, shift work).20 Melatonin has been approved by the U.S. Food and Drug Administration (FDA) to treat circadian rhythm sleep disorder in blind children and adults, but it is unregulated and preparations vary greatly in strength. At higher doses, it causes sleep disruption, daytime fatigue, headache, dizziness, and irritability (Table 6). Little information is available about the safety of long-term use.
| Drug | Daily dosage (mg) | Peak action (hours) | Half-life (hours) | Cost, 30 days (generic)* | Adverse effects and considerations | FDA approved? |
|---|---|---|---|---|---|---|
| Nonbenzodiazepines† | ||||||
| Zolpidem (Ambien) | 5 to 10 | 0.5 | 2 to 3 | $102 | Abdominal pain, rebound insomnia; controlled-release formulation better for sleep maintenance; FDA pregnancy risk category B (not controlled-release formulation); CYP3A4-dependent metabolism | Yes |
| Zaleplon (Sonata) | 5 to 10 | 0.5 | 1 | 103 | Better for sleep maintenance; altered color perception; CYP3A4- dependent metabolism | Yes |
| Eszopiclone (Lunesta) | 2 to 3 | 1 | 4 to 6 | 121 | Unpleasant taste (8 to 24 percent), amnesia, hallucinations, worsening depression; CYP3A4-dependent metabolism | Yes |
| Melatonin receptor agonist | ||||||
| Ramelteon (Rozerem) | 8 to 16 | 0.3 | 2 to 5 | 81 | Suicidal ideation, dizziness, headache, increased prolactin levels; contraindicated with fluvoxamine and liver failure; CYP3A4-, CYP1A2-, and CYP2C9-dependent metabolism | Yes |
| Antihistamines | ||||||
| Diphenhydramine (Benadryl) | 25 to 50 | 1 to 3 | 8 | 5 (1 to 3) | Anticholinergic,‡ CNS depression/stimulation; FDA pregnancy risk category B | Yes |
| Doxylamine (Unisom) | 12.5 to 25 | 2 to 3 | 10 | 8 | Anticholinergic, CNS depression/stimulation; FDA pregnancy risk category A | Yes |
| Hydroxyzine (Vistaril) | 25 to 100 | 2 | 3 to 7 | 37 (9 to 21) | Anticholinergic, CNS depression/stimulation | No |
| Antidepressants | ||||||
| Amitriptyline | 25 to 100 | 2 to 4 | 17 to 40 | 21 (2 to 4) | Anticholinergic, morning sedation, daytime somnolence, accidents, cardiac toxicity, sexual dysfunction, serotonin syndrome (SSRI interaction), exacerbates restless legs syndrome and periodic limb movement; CYP2D6-dependent metabolism | No |
| Trazodone (Desyrel) | 50 to 150 | 0.5 to 2 | 8 | 68 (9 to 13) | Same as above | No |
| Mirtazapine (Remeron) | 15 to 45 | 1.2 to 1.6 | 20 to 40 | 104 (81 to 83) | Anticholinergic, dyspnea, edema, hyper- or hypokinesia, increased appetite | No |
| Rare: facial edema, delusions, hallucinations, seizures, abdominal pain, back pain, agranulocytosis | ||||||
| Benzodiazepines§ | ||||||
| Triazolam (Halcion) | 0.125 to 0.5 | 1 to 2 | 1.5 to 5 | $42 (18 to 21) | Rapid onset, short half-life; anterograde amnesia, rapid eye movement sleep rebound; CYP3A4-dependent metabolism | Yes |
| Estazolam | 1 to 2 | 2 (0.5 to 6) | 10 to 24 | 43 (18 to 27) for 1 mg nightly | Rapid onset, intermediate half-life; daytime sleepiness | Yes |
| Temazepam‖ (Restoril) | 7.5 to 30 | 1.2 to 1.6 | 3.5 to 18.4 | 99 (20 to 21) for 15 mg | Medium onset, intermediate half-life; daytime sleepiness, less effective sleep induction | Yes |
| Herbs and supplements | ||||||
| Melatonin | 0.5 to 10 | 1 | 0.5 to 2 | 3 to 5 for 3 mg | Sleep disruption, daytime fatigue, headache, dizziness, irritability | No |
| Valerian root | 200 to 1,000 | 1 | Not known | 2 to 3 for 450 mg | Daytime sedation, hepatotoxicity | No |
Valerian root causes central sedation by inhibiting the breakdown of γ-aminobutyric acid (GABA) or its metabolites. There is minimal evidence for its effectiveness in treating insomnia.4 Residual daytime sedation and, rarely, hepatotoxicity are adverse effects. Preparations are unregulated by the FDA and may vary in valerian content.
ALCOHOL
At least 10 percent of young adults use OTC medications or alcohol in any given year to improve sleep. Alcohol, a central nervous system (CNS) stimulant and depressant, acts directly on GABA-gated channels reducing sleep-onset latency, but it also increases wakefulness after sleep onset and suppresses rapid eye movement (REM) sleep. Alcohol has the potential for abuse and should not be used as a sleep aid.
BARBITURATES, OPIATES, AND ANTIDEPRESSANTS
Barbiturates function as GABAA brain receptor agonists, decreasing sleep-onset latency and suppressing REM sleep. They are effective in short-term insomnia (i.e., up to two weeks) but lose the ability to induce and maintain sleep beyond this period.21 Longer-term use (i.e., more than two weeks) is associated with tolerance, physical and psychological dependence, and increased adverse effects (i.e., agitation, confusion, nightmares, hallucinations, lethargy, and hangover). Barbiturates should rarely be used as sleep aids.
Opiates fragment sleep and decrease REM and stage 2 sleep. By producing analgesia and sedation, opiates may be appropriate in carefully selected patients with pain-associated insomnia.
Some antidepressants (e.g., amitriptyline, doxepin, trazodone [Desyrel], mirtazapine [Remeron]) produce sedation by blocking acetylcholine, norepinephrine, and serotonin presynaptic receptors. Compared with placebo, antidepressants decrease sleep-onset latency and wakefulness after sleep onset (Table 7).4,13,14,16,22 They also increase total sleep time, sleep efficiency, and sleep quality but suppress REM sleep.4 Antidepressants are an effective treatment option in patients with insomnia and coexisting depression. Trazodone is the most commonly prescribed sleep aid, but there is insufficient evidence to support its use in the absence of depression.
BENZODIAZEPINE HYPNOTICS
Benzodiazepines bind to GABA and GABAA receptors, acting as agonists. They have less risk of overdose and abuse potential than barbiturates. Benzodiazepines increase sleep time and improve sleep quality by reducing sleep-onset latency and wakefulness after sleep onset and by increasing sleep efficiency (Table 7).4,13,14,16,22 However, they also potentiate CNS depression with alcohol or other sedatives. Benzodiazepines that have been approved by the FDA for treating chronic insomnia include estazolam, flurazepam (Dalmane), temazepam (Restoril), quazepam (Doral), and triazolam (Halcion). Rapidly acting drugs with shorter half-lives (i.e., estazolam, triazolam, and temazepam) are preferred. Temazepam has a slower onset of action and is less effective for initiating sleep. Flurazepam and quazepam have half-lives longer than 24 hours.
| Treatment | SOL (minutes) | WASO (minutes) | Sleep efficiency (%)* | Total sleep time increase (minutes) | Sleep quality (SMD) | Risk difference† | |
|---|---|---|---|---|---|---|---|
| Nonpharmacologic13,14 | −15.7 to −24 | −22.5 | — | 24.3 to 32 | — | — | |
| Relaxation4 | −14.5 | −1.6 | 0.4 | 23.0 | 0.37 | — | |
| Cognitive/behavioral4 | −4.6 | −18.2 | 5.5 | 0.7 | 0.38 | — | |
| Exercise16 | −12 | — | — | 42 | — | — | |
| Benzodiazepines4,22 | −4.2 to −16.5 | −23.1 | 6.3 | 39.1 to 61.8 | 0.08 | 0.15 | |
| Estazolam4 | −10.2 | — | — | — | — | — | |
| Flurazepam (Dalmane)4 | −23.1 | −12.4 | — | — | — | — | |
| Temazepam (Restoril)4 | −11.6 | −23.7 | — | — | — | — | |
| Triazolam (Halcion)4 | −19.7 | −40.0 | — | — | — | — | |
| Nonbenzodiazepines4 | −18.1 | −12.6 | 5.9 | 28.0 | 0.48 | 0.05 | |
| Eszopiclone (Lunesta)4 | −16.7 | −25.8 | — | — | — | — | |
| Zaleplon (Sonata)4 | −20.1 | — | — | — | — | — | |
| Zolpidem (Ambien)4 | −12.8 | −8.5 | — | — | — | — | |
| Antidepressants4 | −7.4 | −11.4 | 13.8 | 53.8 | 0.63 | 0.09 | |
| Doxepin4 | −6.7 | — | — | — | — | — | |
| Trazodone (Desyrel)4 | −12.2 | — | — | — | — | — | |
| Melatonin4 | −8.3 | −9.7 | 3.3 | 5.8 | 0.25 | 0.09 | |
| Valerian4 | −1.3 | −8.4 | −0.1 | 0.8 | 1.38 | 0.06 | |
Because tolerance and dependence occur with prolonged use, benzodiazepines are most useful for the short-term treatment of insomnia.22 Using benzodiazepines for more than four weeks increases the likelihood of dependence (defined as a compulsive or chronic need) and withdrawal phenomena (defined as a symptom complex that develops following discontinuation). An estimated 10 to 30 percent of chronic benzodiazepine users develop dependence, and 50 percent suffer withdrawal.23 Dependence is more likely with daily use for more than four months, with higher doses, in older patients, in patients with previous hypnotic or alcohol dependence, and when using high-potency, shorter- acting benzodiazepines.17
Benzodiazepine withdrawal may cause anxiety, depression, nausea, perceptual changes, rebound insomnia, intense dreams, nightmares, and poor memory consolidation. Withdrawal symptoms may develop within a few hours of discontinuing a short-acting benzodiazepine, or up to three weeks after discontinuing a long-acting benzodiazepine.23 Although often recommended, gradually tapering the dose alone is ineffective in achieving long-term discontinuation.24 However, combining CBT with tapering of benzodiazepines results in successful discontinuation in 70 percent of patients at 12 months.24
With short-acting drugs, rebound insomnia can occur the same night the drug is administered, leading to ante-grade memory impairment. Their use increases the risk of motor vehicle collisions, falls and serious injuries, and fatal overdose in older adults.25–27 All benzodiazepines may cause respiratory depression in patients with pulmonary disease.
NONBENZODIAZEPINE HYPNOTICS
The newer nonbenzodiazepines selectively bind to type 1 benzodiazepine receptors in the CNS. Unlike benzodiazepines, the nonbenzodiazepines have minimal impact on sleep stages and no REM sleep rebound. Tachyphylaxis is unusual. Nonbenzodiazepines undergo hepatic degradation, and doses should be reduced in older patients and in those with hepatic dysfunction.28 Direct comparisons of nonbenzodiazepines and benzodiazepines are not available. However, when indirectly compared, the nonbenzodiazepines are similarly effective but have less overall risk of adverse effects.29 Nevertheless, these newer agents can cause impaired memory and psychomotor retardation.30 Nonbenzodiazepine hypnotics approved by the FDA for the treatment of insomnia include zolpidem (Ambien), zaleplon (Sonata), and eszopiclone (Lunesta).
Zolpidem
Zolpidem decreases sleep-onset latency, improves sleep quality, increases stage 2 and slow-wave sleep, and does not exhibit tolerance or rebound following five weeks of continuous use at recommended dosages.4,29 Adverse effects occur at daily dosages of 20 mg or more. Because of its longer half-life, a controlled-release version (Ambien CR) in a dosage of 6.25 to 12.5 mg daily may be better for maintaining sleep, but it should not be readministered following nocturnal awakenings and has not been shown to reduce adverse effects.17 Direct comparisons of zolpidem with controlled-release zolpidem have not been published.
Zaleplon
Zaleplon decreases sleep-onset latency. Its short half-life (i.e., one hour) enables readministration following nocturnal awakenings. It is particularly useful in patients who have trouble falling asleep and maintaining sleep and can be administered up to four hours before the anticipated wake time.17 Zaleplon causes less memory and psychomotor impairment than do benzodiazepines and zolpidem.28 Some patients report visual disturbances, such as a change in color perception.
Eszopiclone
Eszopiclone, an isomer of zopiclone, is the only hypnotic with FDA approval for use longer than 35 days. Eszopiclone has evidence of effectiveness for six months of therapy in a randomized, placebo-controlled trial, although there is some attenuation of its effect over time.31 It produces significant and sustained decreases in sleep-onset latency, wake time, number of awakenings, and number of nights awakened per week; it also improves total sleep time and quality of sleep.31 Eszopiclone has not been directly studied in comparison to other hypnotics or nonpharmacologic therapy. Higher doses (2 to 3 mg) are more effective for sleep maintenance, whereas lower doses (1 to 2 mg) are suitable for difficulty in falling asleep. The onset of action may be delayed if eszopiclone is taken with a high-fat meal. Rare cases of fatal overdose when used with other CNS depressants have been reported.
RAMELTEON (ROZEREM)
Ramelteon is a selective melatonin receptor agonist targeting the melatonin MT1 and MT2 receptors in the brain. It reduces sleep-onset latency and increases sleep periods.32 However, patient evaluations of improvement are inconsistent and there are no comparison studies. Ramelteon has not been studied in patients with depression, anxiety, shift work, or jet lag.33 There is a low likelihood of abuse and physical dependence. Serious adverse effects attributed to ramelteon are rare, affecting less than 1 percent of patients. Common side effects include somnolence, headache, fatigue, nausea, and dizziness. The metabolism of ramelteon is reduced in patients with severe hepatic impairment. Ramelteon is the only non-scheduled drug for insomnia.
COMBINATION THERAPY
There is little evidence to support combining nonpharmacologic and pharmacologic treatments for insomnia. The results of one study that compared benzodiazepine plus CBT versus benzodiazepine alone showed that sleep efficiency was minimally improved with the use of combination therapy.34 Wakefulness after sleep onset and total sleep time were not significantly different. Another study that compared CBT plus zolpidem, CBT alone, zolpidem alone, and placebo found improvements in sleep-onset latency in the CBT-only group. The authors of a third study that compared benzodiazepine plus CBT versus CBT alone found no differences in sleep outcomes.34
Treatment Comparisons
A meta-analysis comparing pharmacologic and nonpharmacologic treatments found similar short-term effectiveness (two to four weeks) in patients with primary insomnia. Nonpharmacologic therapy showed slightly greater reductions in sleep-onset latency, but wakefulness after sleep onset, number of awakenings, total sleep time, and sleep quality were not significantly different.15
There are no direct comparisons of the effectiveness of the newer nonbenzodiazepines and other medications for chronic insomnia. Indirect comparisons in which each medication was compared with placebo are shown in Table 7.4,13,14,16,22 In one meta-analysis, use of nonbenzodiazepines did not significantly reduce sleep-onset latency compared with benzodiazepines; however, a significant reduction was noted when nonbenzodiazepines were compared with antidepressant and melatonin therapies.8 Zolpidem and benzodiazepine therapies were equally effective compared with placebo for patients 65 years or younger.35 In a meta-analysis of patients 60 years and older with insomnia, researchers found that benzodiazepines and nonbenzodiazepines were associated with an increased risk of adverse events.36
The abuse potential of hypnotics is often overstated. Most patients taking hypnotics do so for a short period; only 10 to 15 percent take hypnotics long-term. Although substance abusers may abuse benzodiazepines, they rarely abuse nonbenzodiazepines.37 Hypnotic overdose is generally not life-threatening unless more than one drug in a class or opiates are also ingested.
The cost of nonbenzodiazepines is considerably higher than benzodiazepines. An economic evaluation comparing the cost-effectiveness of nonpharmacologic treatment, benzodiazepines, eszopiclone, and no treatment in older adults found that, compared with benzodiazepines, nonpharmacologic therapy (i.e., CBT) produced a net gain of 0.37 quality-adjusted life-years at a savings of $2,781 over 10 years.38