
Am Fam Physician. 2007;76(4):539-544
Patient information: See related handout on Vibrio vulnificus infection, written by the authors of this article.
Author disclosure: Nothing to disclose.
Vibrio vulnificus infection is the leading cause of death related to seafood consumption in the United States. This virulent, gram-negative bacterium causes two distinct syndromes. The first is an overwhelming primary septicemia caused by consuming raw or undercooked seafood, particularly raw oysters. The second is a necrotizing wound infection acquired when an open wound is exposed to warm seawater with high concentrations of V. vulnificus. Most patients, including those with primary infection, develop sepsis and severe cellulitis with rapid development to ecchymoses and bullae. In severe cases, necrotizing fasciitis can develop. Case-fatality rates are greater than 50 percent for primary septicemia and about 15 percent for wound infections. Treatment of V. vulnificus infection includes antibiotics, aggressive wound therapy, and supportive care. Most patients who acquire the infection have at least one predisposing immunocompromising condition. Physician awareness of risk factors for V. vulnificus infection combined with prompt diagnosis and treatment can significantly improve patient outcomes.
Vibrio vulnificus is a species of gram-negative, motile, curved bacterium that is part of the Vibrio genus and the Vibrionaceae family. Other members of this family include V. cholerae (rare in the United States) and V. parahaemolyticus, both of which cause acute gastrointestinal illness characterized by severe diarrhea. Unlike other members of this family, V. vulnificus infection is extremely invasive. Even with prompt diagnosis and aggressive therapy, the case-fatality rate is 30 to 40 percent.1–3
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Physicians should consider Vibrio vulnificus infection in patients with sepsis and severe skin lesions and should ask them about recent raw oyster consumption. | C | 2, 12–14 | Case series demonstrate high morbidity and mortality with V. vulnificus infection, and physician awareness is recommended. |
| Patients presenting with painful, rapidly progressive hemorrhagic bullae should receive prompt surgical evaluation for possible debridement. | C | 20, 27, 28 | Case series show a benefit from aggressive surgical management of necrotizing soft tissue infections. |
| Patients with chronic liver disease or immunocompromising conditions should avoid eating raw or undercooked seafood and open wound exposure to warm seawater. | C | 4, 29, 30 | Consensus guidelines |
Epidemiology
V. vulnificus is common in warm seawater and thrives in water temperatures greater than 68°F (20°C). The organism is not associated with pollution or fecal waste. The taste, appearance, and odor of seafood are not affected by V. vulnificus contamination, and proper cooking methods readily kill the organism. Although it is found in all coastal waters of the United States, most V. vulnificus infections are attributed to consuming raw oysters harvested in the Gulf of Mexico during the summer.2 Because these oysters are shipped throughout the United States, infections are not limited to endemic areas.4
Approximately 25 percent of V. vulnificus infections are caused by direct exposure of an open wound to warm seawater containing the organism. Exposure typically occurs when the patient is participating in water activities such as boating, fishing, or swimming. Infections are occasionally attributed to contact with raw seafood or marine wildlife.1
V. vulnificus is one of the few foodborne illnesses with an increasing incidence. The Centers for Disease Control and Prevention estimates that the average annual incidence of all Vibrio infections increased by 41 percent between 1996 and 2005.5 In 2004, V. vulnificus was documented in 92 infections; 64 patients with the infection had septicemia, and 28 patients had wound infections.1 These data emphasize the need for physicians to familiarize themselves with the risk factors and clinical characteristics of V. vulnificus infection.
Risk Factors
Table 12 includes risk factors for developing V. vulnificus infection. After the organism enters the body, several factors determine if significant illness develops. Patients with immunocompromising conditions, especially alcoholic liver disease or hepatitis B or C, have a higher risk of infection.3
| Risk factor | Patients with primary septicemia and the risk factor (%) | Patients with a wound infection and the risk factor (%) |
|---|---|---|
| Consumption of raw oysters in the week before becoming ill | 96 | — |
| Wound exposure to warm seawater or raw seafood juice in the week before becoming ill | — | 100 |
| Any chronic disease | 97 | 68 |
| Liver disease | 80 | 22 |
| Alcoholism | 65 | 32 |
| Diabetes | 35 | 20 |
| Malignancy | 17 | 10 |
| Renal disease | 7 | 7 |
Iron overload, documented by high transferrin saturation, is common in patients with liver disease and other immunocompromising conditions who develop V. vulnificus infection.2 In human and animal studies, high levels of free iron have markedly increased the growth and lethality of V. vulnificus.6,7 Patients with chronic liver disease have a much higher risk of septicemia and death; approximately 80 percent of deaths occur in patients with liver disease.2
Several characteristics of the organism facilitate the development of clinical disease. V. vulnificus strains with capsular materials are associated with high bacterial virulence.8 In addition, V. vulnificus produces several extracellular enzymes, including metalloproteinase, lecithinase, lipase, caseinolytic protease, deoxyribonuclease, mucinase, and elastase.9,10 Metalloproteinase destroys basement membrane collagen in blood vessels10 and has fibrinolytic properties11 that cause hemorrhage and edematous skin changes.
Clinical Presentations
Patients with primary septicemia caused by V. vulnificus infection require hospitalization. Characteristic symptoms include fever, diarrhea, nausea, and vomiting. One half of patients have changes in mental status, and almost one third are in septic shock at hospital admission.12 Within 24 hours of symptom onset, more than one half of patients develop the characteristic skin lesions of severe cellulitis with ecchymoses and bullae.12,13 V. vulnificus infection should be considered in patients with sepsis and severe skin lesions, and patients should be asked about raw oyster consumption and seawater exposure.2,12–14
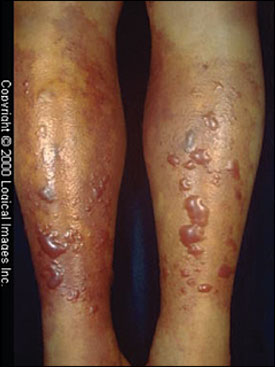
Patients with primary wound infections caused by V. vulnificus develop painful cellulitis that progresses rapidly. Marked local tissue swelling with hemorrhagic bullae is characteristic (Figure 1). Systemic symptoms include fever and chills.12,13 Almost one half of patients develop bacteremia,13,14 more than 10 percent develop hypotension, and almost one third develop changes in mental status.12
Rarely, patients with V. vulnificus infection present with common gastroenteritis.12 V. vulnificus infection should be considered in immunocompromised patients who have recently been exposed to seawater or consumed raw seafood.
Other presentations have occurred less often: infection of mucosal sites and corneal ulcers after handling seafood,15 tubo-ovarian abscesses after sexual activity in seawater,16 and peritoneal infection after receiving dialysis from seawater-contaminated equipment.17 A high index of suspicion is required to diagnose V. vulnificus infection with these rare presentations.
Illustrative Case
An 80-year-old man presented to the emergency department with excruciating pain in his right forearm. He reported spending the previous night fishing in Corpus Christi Bay (Tex.), where he accidentally pierced his right index finger with a live shrimp. Hemorrhagic bullae were present, extending from the hand to the upper arm. He also presented with confusion. His vital signs were a temperature of 100°F (38°C), blood pressure of 88/44 mm Hg, pulse rate of 113 beats per minute, and respiratory rate of 20 breaths per minute. The patient had a history of hypertension, chronic renal failure that did not require dialysis, congestive heart failure, and cirrhosis secondary to alcohol abuse. Laboratory studies revealed a white blood cell count of 6,600 per mm3 (6.6 × 109 per L) with 26 percent bands, hemoglobin level of 13.1 g per dL (131 g per L), platelet count of 33,000 per mm3 (33 × 109 per L), blood urea nitrogen level of 63 mg per dL (22.5 mmol per L), and creatinine level of 4.4 mg per dL (390 μmol per L). A Gram stain of the exudate showed a curved, gram-negative rod. Blood and wound cultures were obtained.
The patient was admitted to the intensive care unit and was treated with oxygen, fluid resuscitation, and intravenous ceftriaxone (Rocephin) and doxycycline (Doxy 100). Within six hours of admission, he required norepinephrine for blood pressure support. By the third day of hospitalization, dialysis was required because of worsening renal failure. On the fourth day of hospitalization, the patient markedly improved, answered questions appropriately, and no longer required pressor support. Wound culture confirmed the clinical diagnosis of V. vulnificus infection. After five days in the intensive care unit, he was in stable condition and was transferred to a local hospital.
Diagnosis
| Infection | Patient history | Underlying conditions | Physical examination findings |
|---|---|---|---|
| Group A Streptococcus species | Skin abrasion, trauma, recent herpes zoster infection, human bite, intravenous drug abuse | Diabetes, cancer, alcoholism, stasis dermatitis | Intense erythema, edema, lymphadenopathy, hemorrhagic and necrotic bullae |
| Staphylococcus aureus | Skin trauma, recent hospitalization or surgery, intravenous drug abuse | Obesity, diabetes, immunocompromising condition | Furuncles, local abscesses, diffuse macular erythroderma |
| Polymicrobial | Diabetic foot ulcer, recent surgery | Diabetes, immunocompromising condition, vascular disease | Moist gangrene with a foul odor |
| Pseudomonas species | Bacteremia, moist skin infection, severe burn, recent hospitalization | Immunocompromising condition | Hemorrhagic and necrotic bullae |
| Vibrio vulnificus | Exposure to raw or undercooked seafood or seawater | Liver disease, immunocompromising condition | Hemorrhagic and necrotic bullae, ecchymoses |
| Clostridium perfringens | Severe trauma with wound contamination, recent surgery, intravenous drug abuse | None | Pale skin, edema, hemorrhagic and necrotic bullae, foul-smelling discharge, gas formation |
| Pasteurella multocida | Cat or dog bite | None | Erythema, edema, serosanguineous discharge, lymphadenitis, tenosynovitis |
| Aeromonas hydrophila | Exposure to freshwater, skin abrasion | Usually none; sometimes an immunocompromising condition | Erythema, bullae, necrosis, possible gas formation |
At hospital admission, laboratory results of patients with V. vulnificus infection are indicative of severe bacterial infection, with a marked left shift in the white blood cell count. Renal injury with a rising serum creatinine level is common.22 With severe V. vulnificus or Streptococcus pyogenes infection, the creatine kinase level is often elevated when necrotizing fasciitis or myonecrosis is present.23
Radiographic studies (e.g., ultrasonography, computed tomography, magnetic resonance imaging) of affected tissues typically show nonspecific changes such as soft tissue edema and pockets of fluid. These findings may help exclude other conditions and guide aspiration attempts and the timing of surgical intervention.
Because sepsis is common, routine blood cultures should be performed when V. vulnificus is suspected. Bullae, ecchymoses, and abscesses are often productive sites to aspirate material for Gram stain and culture. In addition, Gram stain, culture, and frozen-section analysis of tissue is helpful to rapidly visualize bacteria and diagnose necrotizing fasciitis.20 Additional cultures are guided by clinical symptoms and may include ocular, peritoneal, sputum, cervical, and stool cultures. Stool cultures require a thiosulfate citrate bile salts sucrose agar for isolation.24
Treatment and Prognosis
The recommended antibiotic therapy for V. vulnificus infection is doxycycline, 100 mg intravenously or orally (Vibramycin) twice a day; plus ceftazidime (Fortaz), 2 g intravenously every eight hours. Alternative antibiotic therapies are cefotaxime (Claforan), 2 g intravenously every eight hours; or ciprofloxacin (Cipro), 750 mg orally or 400 mg intravenously twice a day.25,26
In addition to antibiotics, many patients require aggressive supportive therapy in the intensive care setting. Aggressive and prompt wound care is essential. Surgical debridement; incision and drainage of abscesses; and, sometimes, amputation have been shown to reduce mortality and shorten hospitalization.20,27,28 Patients presenting with painful, rapidly progressive hemorrhagic bullae should receive prompt surgical evaluation for possible debridement.20,27,28
V. vulnificus infections are commonly fatal, and the prognosis is directly linked to the speed and accuracy of diagnosis and treatment. When treatment was delayed by as little as 24 hours in patients with septicemia, mortality rates increased from 33 to 53 percent. Mortality rates increased to 100 percent in patients who were not treated within 72 hours.12 Recent data show that when all types of V. vulnificus infections are combined, the overall mortality rate is 35 percent.1
Prevention
Table 329,30 includes recommendations for reducing the risk of V. vulnificus infection. Because V. vulnificus–related septicemia is usually caused by consuming raw oysters, most disease can be prevented by not eating this food. Limiting consumption of raw oysters to the winter months also can reduce the risk of infection. Patients with chronic liver disease or immunocompromising conditions are particularly vulnerable to infection and should be advised to avoid raw or undercooked seafood. Persons with open wounds should avoid contact with warm seawater.4,29,30
| Avoid contact with raw seafood juices; use separate cutting boards and knives for seafood and nonseafood | |
| Avoid eating raw oysters or seafood, especially if an immunocompromising condition or chronic liver disease is present; the risk is highest with seafood harvested in the summer | |
| Cook shellfish thoroughly: | |
| In the shell: boil until the shells open, then boil for another five minutes; or steam until the shells open, then steam for another nine minutes (do not eat shellfish that do not open during cooking) | |
| Shucked oysters: boil for at least three minutes, or fry for at least 10 minutes at 375°F (191°C) | |
| Promptly refrigerate leftover seafood | |
| Wear gloves when handling raw oysters or shellfish | |
| Persons with open wounds: | |
| Avoid contact between open wounds and seawater, especially if water temperature is more than 68°F (20°C), or raw seafood | |
| Wash any wound that is exposed to seawater with soap and clean water | |
| Immediately seek medical care for any wound that appears infected | |