
Am Fam Physician. 2008;77(4):483-490
Patient information: See related handout on teenage tobacco use, written by the authors of this article.
Author disclosure: Nothing to disclose.
After steadily decreasing since the late 1990s, adolescent smoking rates have stabilized at levels well above national goals. Experts recommend screening for tobacco use and exposure at every patient visit, although evidence of improved outcomes in adolescents is lacking. Counseling should be provided using the 5-A method (ask, advise, assess, assist, and arrange). All smokers should be offered smoking cessation assistance, including counseling, nicotine replacement therapy, bupropion therapy, or combination therapy. Pharmacotherapy of any kind doubles the likelihood of successful smoking cessation in adults; however, nicotine replacement therapy is the only pharmacologic intervention that has been extensively studied in children. Community interventions such as smoking bans and educational programs have been effective at reducing smoking rates in children and adolescents. Antismoking advertising and tobacco sales taxes also help deter new smokers and motivate current smokers to attempt to quit.
Tobacco is the leading cause of preventable death in the United States, causing more than 440,000 deaths annually.1 An additional 8 million Americans have smoking-related diseases such as cancers of the lungs, larynx, oral cavity, and esophagus, as well as pulmonary diseases such as chronic obstructive pulmonary disease (COPD).1 Tobacco is responsible for an estimated $157 billion in annual health care expenditures.1
Of greater concern is the large number of adolescents who start smoking early in life and continue throughout adulthood. Currently, 3 million U.S. adolescents younger than 18 years smoke cigarettes.2 Almost one fourth of adolescents smoke by the time they graduate from high school, and almost 90 percent of adults who smoke began at or before age 18.2 Each day about 4,400 teenagers try their first cigarette and contribute to the 1.5 million adolescents who begin to smoke each year.3 A significant number of adolescents who use illicit drugs smoked cigarettes first.4
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Screening for tobacco use in adolescents is recommended at each physician visit, although the U.S. Preventive Services Task Force has found the evidence insufficient to recommend for or against routine screening in this age group. | B | 8, 19, 31 |
| Tobacco cessation should be offered regularly using the 5-A method (ask, advise, assess, assist, and arrange). | C | 9 |
| Nicotine replacement therapy is recommended for adolescents who meet criteria for tobacco dependence. | B | 15–18, 20 |
Smoking rates among high school students peaked in 1976, when nearly 40 percent of graduating seniors classified themselves as current smokers. Rates tapered off in the 1980s but then steadily increased, peaking again in 1997.5 The Tobacco Master Settlement Agreement (MSA) was reached in 1998 between state attorneys general and the major tobacco manufacturers; the settlement banned certain types of tobacco advertising targeted at teenagers, and it launched a national antismoking campaign and several state-level campaigns. At the same time, cigarette prices increased substantially as a result of higher state taxes and the tobacco companies' efforts to recover money lost in the MSA.
All of these factors have contributed to a significant decrease in adolescent smoking rates. Unfortunately, this downward trend has recently stalled and is showing signs of reversal, with current rates well above the Healthy People 2010 objective of 16 percent or less.2 Tobacco use among 10th and 12th graders has slightly increased, from 21.9 to 23.0 percent between 2003 and 2005.2,5
Risk Factors
Many factors, internal and external, affect the risk for adolescent smoking. White adolescents consistently smoke much more than their Hispanic and black counterparts. Sex does not appear to exert an influence, with male and female smoking rates remaining equivalent over the past decade.2 Educational goals play a role, because students who plan to attend a four-year college have significantly lower smoking rates than those who do not.3 Finally, stress and psychiatric disorders such as attention-deficit/hyperactivity disorder and depression have been linked to increased adolescent smoking rates.6,7 External risk factors include peer pressure, parental smoking, media advertising, and beliefs about the positive and negative consequences of smoking.3
Assessment
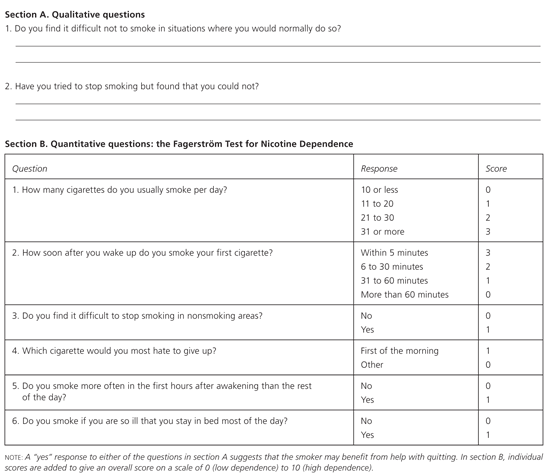
Although the U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against routine screening for tobacco use or interventions to prevent and treat tobacco use and dependence among adolescents,8 the U.S. Public Health Service and the Institute for Clinical Systems Improvement (ICSI) recommend that physicians determine and document tobacco use and exposure to secondhand smoke at every office visit.9,10 Tobacco cessation using the 5-A method (ask, advise, assess, assist, and arrange) should be offered on a regular basis to all adolescents who smoke (Table 1).9 After an adolescent has been identified as a smoker, the physician can assess the level of tobacco dependence using either qualitative or quantitative methods (Figure 1).11
| Ask about tobacco use at each appointment |
| Advise all adolescents who are smoking to stop |
| Assess adolescent's willingness to attempt to quit |
| Assist efforts to quit |
| Arrange reliable follow-up |
A major barrier to screening is the fact that many adolescents do not visit a physician on a regular basis. Therefore, sports or school physical examinations offer an excellent opportunity to assess for smoking and offer help with cessation. Adolescents are unlikely to volunteer the fact that they smoke unless they are asked directly. To facilitate disclosure, it may be wise to ask when the parent or guardian is not present.
Involving the whole family in treatment can be beneficial. Offering smoking cessation services to parents who also smoke will limit the adolescent's exposure to secondhand smoke, as well as remove a source of temptation in the home.
Treatment
The ICSI recommends aggressive intervention for smoking cessation in mature adolescents (i.e., 16 years and older), including community interventions, formal tobacco cessation programs, and pharmacotherapy.10 The USPSTF found limited evidence to support the effectiveness of counseling children and adolescents in the primary care setting, but it found that school and classroom smoking cessation programs are more effective than no intervention.8
Therapies for adolescents include counseling, nicotine replacement therapy, psychoactive medication (e.g., bupropion [Zyban]), and combination therapy. Pharmacotherapy should be explained and offered to all patients with nicotine dependence (Table 212–18 ).10 Most studies on smoking cessation have been conducted in adults.3 The nicotine patch (Nicoderm), nicotine gum (Nicorette), and bupropion are the only therapies that have been studied in adolescents. Most studies in adolescents have shown significant reductions in the number of cigarettes smoked daily, but low overall abstinence rates.19 Pharmacotherapy of any kind doubles the probability of smoking abstinence in adults.10
| Therapy | Availability | Dosage | Prescribing instructions | Precautions | Side effects | Studied in adolescents? | Cost (generic)* |
|---|---|---|---|---|---|---|---|
| Bupropion (Zyban) | Rx | 150 mg daily for 3 days, then twice daily for 7 to 12 weeks | Stop smoking one week after starting treatment Avoid or minimize alcohol intake Do not cut, crush, or chew tablets Separate doses by at least 8 hours to avoid insomnia; take last dose before 6 p.m. | FDA pregnancy category B Use caution in patients with seizure disorders, eating disorders, alcoholism, or uncontrolled hypertension, and in those who have taken a monoamine oxidase inhibitor within the past 14 days | Seizures, arrhythmias, Stevens- Johnson syndrome, erythema multiforme, hallucinations, mania, suicidality, hypertension, migraines | Yes; safe and mildly effective as monotherapy. Not as effective when used in combination with nicotine replacement therapy12–14 | $174 ($116) per month |
| Nicotine gum (Nicorette) | OTC | 1 piece every 1 to 2 hours for 6 weeks, then every 2 to 4 hours for 3 weeks, then every 4 to 8 hours for 3 weeks 2-mg gum should be used by patients who smoke ≤ 25 cigarettes per day (maximum, 30 pieces per day) 4-mg gum should be used by patients who smoke > 25 cigarettes per day (maximum, 20 pieces per day) | Chew to activate taste, then hold between mouth and gum for 30 minutes or until taste disappears | FDA pregnancy category D Use caution in patients with cardiovascular disease, arrhythmias, or worsening angina, and in those with a history of myocardial infarction within the past two weeks | Mouth soreness, hiccups, heartburn, jaw pain, tooth disorders, headache, nausea | Yes; effective when combined with counseling15 | $47 for 110 pieces ($42 to 47) |
| Nicotine inhaler (Nicotrol Inhaler) | Rx | 6 to 16 cartridges daily (1 cartridge = 4 mg) After initial therapy for 6 to 12 weeks, taper over 6 to 12 weeks | Stop smoking at onset of treatment Avoid acidic beverages Use frequent, continuous puffing for 20 minutes per cartridge | Same as above Use caution in patients with asthma | Bronchospasm, mouth or throat irritation, cough, headache, rhinitis, dyspepsia, taste changes | No | $133 for 168 cartridges es |
| Nicotine lozenge (Commit) | OTC | 1 piece every 1 to 2 hours for 6 weeks, then every 2 to 4 hours for 3 weeks, then every 4 to 8 hours for 3 weeks 2-mg lozenges should be used by patients who smoke their first cigarette > 30 minutes after awakening 4-mg lozenges should be used by patients who smoke their first cigarette ≥ 30 minutes after awakening | Dissolve in mouth over 20 to 30 minutes Do not chew, bite, or swallow Do not eat or drink for 15 minutes before and after use | Same as above | Headache, diarrhea, heartburn, hiccups, flatulence, nausea, sore throat | No | $78 for 192 2-mg lozenges |
| Nicotine nasal spray (Nicotrol NS) | Rx | 1 or 2 sprays in each nostril every hour (maximum, 80 sprays daily for both nostrils) After initial therapy for 8 weeks, taper over 4 to 6 weeks | Stop smoking at onset of treatment Deliver spray with head tilted slightly back Do not sniff, swallow, or inhale spray | Same as above | Bronchospasm, nasal or throat irritation, watery eyes, sneezing, cough, headache, rhinitis | No | $148 for four 10-mL bottles |
| Nicotine patch (Nicoderm, Nicotrol) | OTC | Nicoderm Patients who smoke 6 to 10 cigarettes per day: 14 mg daily for 6 weeks, then 7 mg daily for 2 weeks Patients who smoke more than 10 cigarettes per day 21 mg daily for 6 weeks, then 14 mg daily for 2: weeks, then 7 mg daily for 2 weeks Nicotrol Patients who smoke 6 to 10 cigarettes per day: 10 mg daily for 6 weeks, then 5 mg daily for 2 weeks Patients who smoke more than 10 cigarettes per day 15 mg daily for 6 weeks, then 10 mg daily for: 2 weeks, then 5 mg daily for 2 weeks | Stop smoking at onset of treatment Place patch on hairless location of upper body or outer arm; rotate sites Nicoderm CQ or generic: remove after 16 to 24 hours Nicotrol: remove every night | Same as above | Skin irritation, headache, nausea, insomnia | Yes; safe and effective as monotherapy or when combined with counseling; not as effective as in adult smokers14,16–18 | $63 for 28 14-mg patches ($60) |
| Varenicline (Chantix) | Rx | 0.5 mg daily for 3 days, then 0.5 mg twice daily for 4 days May continue for an additional 12 weeks if treatment is successful | Stop smoking after 7 days of treatment Take with food | FDA pregnancy category Adjust dosage in renal-impaired patients Not approved for use in patients younger than 18 years | Nausea, insomnia, headache, abnormal dreams, constipation, flatulence, headache, vomiting | No | $108 for 56 pills |
COUNSELING
Studies of counseling interventions have been promising. In one retrospective cohort study involving an initial 45-minute consultation and subsequent telephone follow-up with a tobacco treatment counselor, 18 percent of adolescents who previously smoked were abstinent at six months, and 11.5 percent were abstinent after five years.20 These percentages are significantly higher than those in previous observational studies of adolescent smokers. Several studies have shown increased cessation rates after cognitive behavior therapy.21 A recent study of more than 2,500 adolescents found that a multipart tobacco intervention increased abstinence rates even up to two years later.22 Although such labor-intensive interventions may be impractical during a brief office visit, even interventions of five minutes or less can improve smoking cessation rates.9
NICOTINE REPLACEMENT THERAPY
Adult trials using the nicotine patch commonly report abstinence rates of up to 30 percent at six to 12 months.10 The reason for this discrepancy is unclear. Another study demonstrated higher cessation rates when nicotine replacement therapy was combined with intensive cognitive behavior therapy.15,18 Larger studies are needed to fully examine the potential of nicotine replacement therapy in adolescents.
Most side effects of nicotine replacement therapy are mild and include local skin reactions in patients using the nicotine patch (50 percent); mouth soreness, hiccups, and dyspepsia in those using nicotine gum and lozenges (25 percent); and local throat and nose irritation in those using the nicotine inhaler (40 percent) and nasal spray (90 percent). Caution is advised when using nicotine replacement therapy in pregnant women and in patients with cardiovascular disease.
OTHER MEDICATIONS
No psychoactive medications are currently approved by the U.S. Food and Drug Administration for tobacco cessation in adolescents. Recent reports of increased suicidality in adolescents taking antidepressants have further dampened enthusiasm for this approach.23,24 Bupropion is one of the most effective treatments for tobacco use in adults, resulting in cessation rates of 23 to 38 percent at 12 months.10,25,26 Several studies have shown that bupropion is safe and effective in adolescents, resulting in cessation rates of up to 27 percent at six months.12–14
Common side effects of bupropion therapy include insomnia (35 to 40 percent) and dry mouth (10 percent). Bupropion should not be prescribed to patients with a history of seizures or eating disorders, or to patients who are taking monoamine oxidase inhibitors.10
Selective serotonin reuptake inhibitors (SSRIs), nortriptyline (Pamelor), and clonidine (Catapres) also have been studied for efficacy in smoking cessation. SSRIs have not been proven effective, whereas nortriptyline and clonidine are effective but have more side effects compared with bupropion.10,25,27,28
Varenicline (Chantix), a recently approved nicotinic receptor antagonist, is not labeled for use in adolescents.
COMBINATION THERAPY
Studies of counseling in combination with pharmacotherapy in adolescent smokers show improved abstinence rates.10,15,18,20,21 In adults, the combination of nicotine patches with most other forms of nicotine replacement therapy (gum, lozenges, or spray, but not inhalers) is more effective than any replacement method alone.10,16 Combining bupropion and nicotine replacement therapy results in improved abstinence rates compared with either therapy alone, but the difference is not statistically significant.26 A study of 211 adolescent smokers randomly assigned to the nicotine patch plus bupropion found no statistically significant treatment effect compared with the nicotine patch plus placebo.14
Community Interventions
EDUCATION
There is limited evidence that school- and community-based education programs significantly decrease smoking rates. School-based prevention programs typically have short-term, but not long-term, effects on adolescents.29 Successful programs are grade-, age-, and culture-appropriate and focus on the immediate consequences of smoking and on coping strategies.19 The American Academy of Family Physicians' Tar Wars Program, which is targeted to fourth- and fifth-grade students, has been shown to effectively educate children about the consequences of tobacco use.30 Education programs are most effective when combined with strong antismoking policies at the schools and are part of a comprehensive local or state tobacco cessation effort. Programs based solely on social influences are not effective.31
ADVERTISING
Tobacco advertising is a significant contributor to adolescent smoking. Despite abolition of broadcast media and billboard advertising, tobacco advertisement continues in magazines and through promotional activities such as sponsorship of sporting events. Anti-tobacco counter-advertising at the local, state, and national levels can help counteract this influence. Evidence shows that anti-tobacco media campaigns, especially paid television advertising, have significantly decreased tobacco use in adolescents since 1998.32 A dose-response relationship has been found in the number of exposures to advertising and adolescents' ability to recall them. Exposure to at least one state-funded anti-tobacco advertisement in the previous four months is associated with lower perceived rates of friends' smoking and a greater perceived harm of smoking.33
OTHER ANTISMOKING STRATEGIES
Smoking bans at home and at school can effectively decrease adolescent smoking rates. Smoke-free school zones decrease smoking rates by up to 40 percent when the ban is implemented and enforced.34 Smoking bans in the home lead to a perception of lower smoking prevalence and decreased smoking acceptability, which can reduce smoking initiation.35
Price is another important variable, with multiple studies showing that increased tobacco prices are effective in decreasing tobacco use in adolescents and adults.36
Access to tobacco also clearly factors into adolescent smoking. More than 500 Web sites sell tobacco products; many of these sites offer reduced prices by avoiding tobacco and sales taxes, and many have lax age verification.37 Before the passage of the PACT (Prevent All Cigarette Trafficking) Act in 2003, children as young as 11 were more than 90 percent successful in purchasing cigarettes online.37