
Am Fam Physician. 2008;77(10):1423-1430
A more recent article on primary brain tumors in adults is available.
Author disclosure: Nothing to disclose.
Primary malignant brain tumors account for 2 percent of all cancers in U.S. adults. The most common malignant brain tumor is glioblastoma multiforme, and patients with this type of tumor have a poor prognosis. Previous exposure to high-dose ionizing radiation is the only proven environmental risk factor for a brain tumor. Primary brain tumors are classified based on their cellular origin and histologic appearance. Typical symptoms include persistent headache, seizures, nausea, vomiting, neurocognitive symptoms, and personality changes. A tumor can be identified using brain imaging, and the diagnosis is confirmed with histopathology. Any patient with chronic, persistent headache in association with protracted nausea, vomiting, seizures, change in headache pattern, neurologic symptoms, or positional worsening should be evaluated for a brain tumor. Magnetic resonance imaging is the preferred initial imaging study. A comprehensive neurosurgical evaluation is necessary to obtain tissue for diagnosis and for possible resection of the tumor. Primary brain tumors rarely metastasize outside the central nervous system, and there is no standard staging method. Surgical resection of the tumor is the mainstay of therapy. Postoperative radiation and chemotherapy have improved survival in patients with high-grade brain tumors. Recent developments in targeted chemotherapy provide novel treatment options for patients with tumor recurrence. Primary care physicians play an important role in the perioperative and supportive treatment of patients with primary brain tumors, including palliative care and symptom control.
Primary malignant brain tumors are rare, accounting for approximately 2 percent of all cancers in U.S. adults. The American Cancer Society estimates that there are more than 18,000 new diagnoses of brain and nervous system cancers causing more than 12,000 deaths each year in the United States.1 Data from the Surveillance, Epidemiology, and End Results program showed an age-adjusted incidence of 6.4 per 100,000 person-years in 2003 compared with 5.85 per 100,000 person-years in 1975.2 The incidence of brain tumors is higher in men than in women (7.6 versus 5.3 per 100,000 person-years),3 and the lifetime risk of developing a brain tumor is 0.65 percent in men and 0.5 percent in women.2 The incidence of brain tumors peaks between 65 and 79 years of age. The incidence of glioblastoma in white persons is approximately double that in black persons.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Patients with symptoms suggesting a brain tumor should be evaluated with gadolinium-enhanced magnetic resonance imaging. | C | 15 |
| Surgery is the treatment of choice for primary brain tumors, especially for resectable high-grade gliomas. | C | 21–24, 38 |
| Radiation with temozolomide (Temodar) therapy improves survival in patients with malignant gliomas. | B | 28, 29, 31 |
Risk Factors
Several central nervous system (CNS) tumors are associated with rare genetic conditions, most commonly the autosomal dominant disorder neurofibromatosis 1. Patients with this disorder have a number of dermatologic manifestations and are at increased risk of optic gliomas and astrocytomas. Although several environmental factors have been associated with brain tumors, exposure to high-dose ionizing radiation is the only proven risk factor.4 Studies of other environmental factors such as occupational exposures, electromagnetic fields, pesticides, cellular telephones, head trauma, and N-nitroso compounds have had inconclusive results.5 Table 1 presents possible risk factors for primary brain tumors.4,5
| Environmental | |
| Proven | |
| High-dose ionizing radiation | |
| Unproven | |
| Alcohol use | |
| Cellular telephones | |
| Chemical agents (e.g., hair dyes, solvents, pesticides, traffic-related air pollution) | |
| Extremely low-frequency electromagnetic fields | |
| Head trauma or injury | |
| Infections (e.g., viruses, Toxoplasma gondii, in utero influenza, varicella) | |
| Nitrosamine, nitrosamide, nitrite, nitrate, or aspartame consumption | |
| Occupational exposures (e.g., rubber, vinyl chloride, petroleum) | |
| Tobacco use | |
| Genetic | |
| Li-Fraumeni syndrome (P53 mutation) | |
| Multiple endocrine neoplasia type 1 | |
| Neurofibromatosis 1 and 2 | |
| Nevoid basal cell carcinoma Syndrome | |
| Tuberous sclerosis | |
| Turcot's syndrome | |
| Von Hippel-Lindau disease | |
Classification
The World Health Organization classifies primary brain tumors based on cellular origin and histologic appearance (Table 2).6 Neuroglial tumors account for more than 80 percent of primary brain tumors and derive from astrocytes, oligodendrocytes, or ependymal cells. Gliomas are divided into four grades; grades I and II tumors are low grade, whereas grades III and IV tumors are high grade.7 Glioblastoma multiforme is the most common type of glioma. Meningiomas derive from meningothelial cells and comprise about 20 percent of primary brain tumors. Primary CNS lymphoma, which has been increasing in the United States, typically occurs in patients with immunodeficiency syndromes, particularly acquired immunodeficiency syndrome.8
| Neuroepithelial tumors | |
| Astrocytic tumors | |
| Pilocytic astrocytoma (grade I) | |
| Subependymal giant cell astrocytoma (grade I) | |
| Diffuse astrocytoma (grade II) | |
| Pleomorphic xanthoastrocytoma (grade II) | |
| Anaplastic astrocytoma (grade III) | |
| Glioblastoma (grade IV) | |
| Oligodendroglial tumors | |
| Oligodendroglioma (grade II) | |
| Anaplastic oligodendroglioma (grade III) | |
| Oligoastrocytic tumors | |
| Oligoastrocytoma (grade II) | |
| Anaplastic oligoastrocytoma (grade III) | |
| Ependymal tumors (grades I to III) | |
| Choroid plexus tumors (grades I to III) | |
| Other neuroepithelial tumors | |
| Angiogenic glioma (grade I) | |
| Chordoid glioma of the third ventricle (grade II) | |
| Neuronal and mixed neuronal-glial tumors (grades I to III) | |
| Pineal tumors (grades I and IV) | |
| Embryonal tumors (grade IV) | |
| Tumors of cranial and paraspinal nerves | |
| Schwannoma (grade I) | |
| Neurofibroma (grade I) | |
| Perineurioma (grades I to III) | |
| Malignant peripheral nerve sheath tumor (grades II to IV) | |
| Tumors of the meninges | |
| Meningioma (grade I) | |
| Atypical meningioma (grade II) | |
| Anaplastic meningioma (grade III) | |
| Lymphomas and hematopoietic neoplasms | |
| Malignant lymphoma (low and high grade) | |
| Plasmacytoma | |
| Granulocytic sarcoma | |
| Other | |
| Germ cell tumors | |
| Tumors of the sellar region (grade I) | |
Diagnosis
CLINICAL PRESENTATION
Presenting signs and symptoms in patients with primary brain tumors (Table 39 ) can be generalized or focal. In the initial stages of disease (low-grade tumors), most symptoms are focal. Generalized symptoms occur with increased tumor size. Common generalized symptoms include headache, nausea, vomiting, seizures, and altered mental functions (e.g., personality changes).9
| Sign or symptom | Percentage with the sign or symptom |
|---|---|
| Headache | 56 |
| Memory loss | 35 |
| Cognitive changes | 34 |
| Motor deficit | 33 |
| Language deficit | 32 |
| Seizures | 32 |
| Personality change | 23 |
| Visual problems | 22 |
| Changes in consciousness | 16 |
| Nausea or vomiting | 13 |
| Sensory deficit | 13 |
| Papilledema | 5 |
In one large study of patients with primary brain tumors, 77 percent of patients reported a dull tension-type headache.10 Two large studies of patients with high-grade gliomas also showed that headache was the most common initial presenting symptom.11,12 In about 50 percent of patients, the headache is persistent and can last more than six months. Headache is often associated with other symptoms, including seizures (50 percent of patients), visual disturbances (40 percent), and nausea and vomiting (38 percent).10 Any patient with chronic, persistent headache in association with protracted nausea, vomiting, seizures, change in headache pattern, neurologic symptoms, or positional worsening should be evaluated for a brain tumor.
Seizure disorder is most common in patients with low-grade gliomas. The type of seizure and associated neurologic symptoms vary with tumor location (Table 413). Seizures can present with aura and postictal symptoms. In two large studies, 18 percent of patients with glioblastoma multiforme initially presented with seizures.11,12
| Tumor location | Signs and symptoms |
|---|---|
| Frontal lobe | Dementia, personality change, gait disturbance, expressive aphasia, seizure |
| Parietal lobe | Receptive aphasia, sensory loss, hemianopia, spatial disorientation |
| Temporal lobe | Complex partial or generalized seizure; behavior change, including symptoms of autism, memory loss, and quadrantanopia |
| Occipital lobe | Contralateral hemianopia |
| Thalamus | Contralateral sensory loss, behavior change, language disorder |
| Cerebellum | Ataxia, dysmetria, nystagmus |
| Brain stem | Cranial nerve dysfunction, ataxia, papillary abnormalities, nystagmus, hemiparesis, autonomic dysfunction |
One third of patients with high-grade tumors initially present with nausea and vomiting, often in association with other symptoms, such as headache and seizures.10 Cognitive dysfunction also may be the initial symptom in patients with brain tumors. Symptoms of cognitive dysfunction include changes in memory, attention, orientation, language abilities, executive function, personality, and daily activities (e.g., sleep, appetite). These symptoms could be caused by the tumor itself, tumor-related epilepsy, or treatment such as surgery, chemotherapy, corticosteroids, radiotherapy, and antiepileptics.
Symptoms of cognitive dysfunction are more common in patients with low-grade gliomas because of prolonged survival and cumulative treatment-related effects during this time. Tumors originating in dominant hemispheres of the brain are more likely to be associated with cognitive dysfunction than those originating in nondominant hemispheres. Cognitive dysfunction is usually progressive, even after aggressive treatment of the tumor. Symptoms related to cognitive dysfunction can be confused with depression, leading to a delay in the diagnosis.14
In addition to a thorough medical history and physical examination, funduscopy and a focused neurologic examination evaluating mental status; cranial nerves; motor, sensory, and cerebellar functions; and deep tendon reflexes should be performed.
DIAGNOSTIC NEUROIMAGING
Diagnosis begins with appropriate brain imaging, followed by histopathology to confirm the diagnosis. Several imaging modalities can be helpful when performing the initial work-up and follow-up and in the evaluation and treatment of brain tumors (Table 5).15–20 With recent advances in structural and functional brain imaging techniques, physicians can determine tumor location and biologic activity. These techniques are also used to assess the effects of treatment, differentiate tumor recurrence from radiation necrosis, and determine tumor progression.
| Modality | Uses |
|---|---|
| CT | Localizing the tumor and defining its dimensions, morphology |
| MRI | Localizing the tumor and surrounding structures with a high-resolution image, diagnosis of supra- and subtentorial tumors, diagnosis of extra- and intra-axial tumors, presurgical planning with three-dimensional imaging, stereotactic biopsy, radiotherapy |
| DTI | Establishing spatial relationships between tumor border and white matter, assessing the progression and regression of white matter tracts caused by tumor growth or resection |
| fMRI | Neurosurgical planning and neurologic risk assessment by localizing the cortical regions that control language, motor, and memory functions |
| MRA | Understanding tumor vascularity and identifying the anatomic relationship between the tumor and blood vessels |
| MRS | Obtaining biochemical and metabolic information about the tumor, determining tumor type and grade by assessing the cellular contents, differentiating tumor from radiation necrosis |
| PET | Metabolic assessment of tumor aggressiveness (grade), assessing the highly metabolic areas within the tumor, differentiating between tumor recurrence and radiation necrosis, functional localization of cortical regions, predicting patient survival and prognosis |
Gadolinium-enhanced cranial magnetic resonance imaging (MRI) is the preferred study for the initial anatomic evaluation of brain tumors.15 Most brain tumors are hypo-intense on T1-weighted images (Figure 1A) and hyperintense on fluid-attenuated inversion recovery (Figure 1B), T2-weighted, and proton-weighted images. MRI is superior to conventional computed tomography (CT) because it can produce higher resolution images and assess lesions in the posterior fossa and spine15; there is also negligible risk of allergic reaction to contrast agents.16
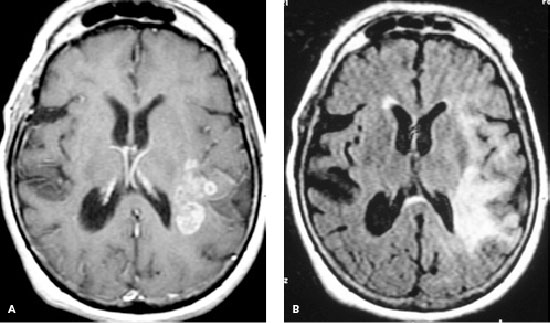
Magnetic resonance spectroscopy provides biochemical and metabolic information about tumors and the normal brain.17 Blood oxygen level–dependent functional MRI is used for neurosurgical planning and neurologic risk assessment in patients with brain tumors.18 Positron emission tomography (PET), a non-invasive and functional imaging technique, is used in assessing diagnosis, grading cerebral gliomas, and differentiating between tumor recurrence and radiation necrosis.19 PET can also be used to predict the response to chemotherapy versus radiochemotherapy.20
STAGING
There is no standard staging system for primary brain tumors, which spread to other parts of the brain and spinal cord through cerebrospinal fluid. Involvement of cerebrospinal fluid (e.g., medulloblastoma, ependymoma) can be determined by analyzing the fluid. Distant metastasis outside the CNS is rare.
Treatment
SURGERY
A comprehensive neurosurgical evaluation is necessary to obtain tissue for diagnosis or for possible resection of the tumor. Surgery is the treatment of choice for a primary brain tumor if the patient is a candidate for complete resection.21–24 Surgery is often curative for intracranial tumors that occur outside the brain, such as pituitary adenomas, schwannomas, and meningiomas.
The decision to perform a complete resection is based on the location and extent of the tumor, histopathology, and comorbid conditions. Patients with asymptomatic meningiomas can be followed up clinically with serial CT or MRI scans every two to three years. A significant increase in meningioma size on a scan or the presence of neurologic symptoms requires neurosurgical evaluation. In patients with high-grade gliomas, a near-total resection is important in decreasing the tumor burden, relieving intracranial pressure, and improving survival.21–24 Near-total resection of low-grade gliomas is not possible because of their slow growth and vague margins.
RADIATION THERAPY
The main types of external beam radiation are conventional and stereotactic radio-surgery. Standard external beam radiation therapy consists of 25 to 35 daily treatments administered over five to seven weeks. The total dose depends on the histology, grade, and location of the tumor and on the extent of resection. Stereotactic radiosurgery often delivers a single high dose of radiation in a one-day session, but it could be administered in two or three large doses. Currently, stereotactic radiosurgery can be used to palliate small, well-demarcated volumes of recurrent glioblastoma multiforme and as a boost after conventional external beam radiotherapy. Its use in the treatment of newly diagnosed malignant gliomas is controversial. Interstitial radiotherapy (brachytherapy) involves surgically implanting radioactive material directly inside the tumor.
CHEMOTHERAPY
Recent advances in chemotherapy have led to a meaningful role in the management of primary brain tumors. Combining temozolomide (Temodar) therapy with radiation improves survival in patients with high-grade gliomas.28,29 A phase III trial of patients with newly diagnosed glioblastoma found that daily, low-dose temozolomide plus standard radiation followed by standard-dose temozolomide improves survival compared with standard radiation alone (median survival was 14.6 versus 12.1 months).28
The methyl guanine and methyl transferase (MGMT) gene is becoming increasingly important in the treatment of glioblastoma multiforme. The MGMT gene encodes for a protein with alkyltransferase activity; this protein leads to the tumor-resistant effects of alkylating and methylating agents. Silencing of the MGMT gene has been shown to be a powerful independent predictor of increased survival.29
The chemotherapy agent irinotecan (Camptosar) and targeted agents such as bevacizumab (Avastin), which targets the vascular endothelial growth factor; and gefitinib (Iressa), erlotinib (Tarceva), and imatinib (Gleevec), which target the epidermal and platelet-derived growth factor receptors, have shown some promise in the treatment of recurrent malignant gliomas.30 Oligodendrogliomas with 1p/19q chromosome deletions respond well to radiotherapy and chemotherapy.31
| Tumor | Treatment |
|---|---|
| Astrocytoma, anaplastic | Surgery and radiation |
| Astrocytoma, high grade | Surgery and radiation, optional chemotherapy |
| Astrocytoma, noninfiltrating | Surgery, optional radiation |
| Brain stem glioma | Radiation |
| Craniopharyngioma | Surgery, optional radiation |
| Ependymoma | Surgery, optional radiation |
| Ependymoma, anaplastic | Surgery and radiation |
| Glioblastoma multiforme | Surgery, radiation, and chemotherapy |
| Medulloblastoma | Surgery, optional radiation |
| Meningioma | Surgery, optional radiation |
| Meningioma, malignant | Surgery and radiation |
| Mixed glioma | Surgery and radiation, optional chemotherapy |
| Oligodendroglioma | Surgery, optional radiation |
| Oligodendroglioma, anaplastic | Surgery and radiation, optional chemotherapy |
| Pineal parenchymal tumor | Surgery and radiation, optional chemotherapy |
| Primary CNS lymphoma | Radiation and chemotherapy |
PERI-AND POSTOPERATIVE TREATMENT
Close postoperative follow-up is important in recognizing and treating complications and tumor recurrence. A repeat MRI with contrast media should be performed within the three days after surgery to determine the extent of tumor resection. Steroids are often the cornerstone treatment in patients with vasogenic edema. In some instances, it is necessary to slowly taper steroid therapy over many months because of symptoms secondary to the residual tumor, even after surgery. It is essential not to overlook adverse effects from steroid therapy, including cognitive impairment, hyperglycemia, gastrointestinal problems, myopathy, and opportunistic infections (e.g., Pneumocystis jiroveci [previously known as Pneumocystis carinii] pneumonia). Steroid withdrawal syndrome has also occurred in patients with myalgias and arthralgias during steroid tapering; this can be confused with rheumatoid arthritis or worsening of the underlying malignancy.33
Although seizures are common during the entire course of the disease, there is no evidence to support routine use of prophylactic anticonvulsants.34,35 If a patient is placed on anticonvulsants postoperatively, it is appropriate to taper the dosage and discontinue use after the first week, particularly in patients who are medically stable.35
Patients with brain tumors, especially glioblastoma multiforme and primary CNS lymphoma, are at increased risk of venous thromboembolism (VTE); the risk is even higher after craniotomy. Whether to use anticoagulation in a patient with a brain tumor who develops VTE is complicated. It is generally believed that controlled anticoagulation with warfarin (Coumadin) or low-molecular-weight heparin is safer and causes fewer complications than inferior vena cava filters.36,37 Primary prophylaxis for VTE using anticoagulation is not well established and is generally not recommended.
Prognosis
Data from the Glioma Outcomes Project revealed that tumor grade, patient age and functional status, and complete resection were prognostic factors (Table 722,38,39 ) for survival in patients with recently diagnosed malignant gliomas.38 Age of 60 years or younger and a score of 70 or greater on the Karnofsky performance scale were associated with prolonged survival in patients with grade III or IV gliomas.
| Age younger than 60 years |
| Patient presents with seizure |
| Tumors in the frontal lobe |
| Low-grade tumor |
| Absence of tumor necrosis |
| Low proliferative activity of tumor cells |
| Karnofsky performance score greater than 70 (patient is able to care for him- or herself) |
| Total or near-total resection |
| Presence of MGMT gene promoter hypermethylation |
Patients with near-total resection also have improved survival. Patients presenting with seizure have a more favorable prognosis, likely because of early diagnosis. Patients with longer duration of disease before diagnosis, indicating low proliferative activity of the tumor cells (low-grade tumor), also have a better prognosis. Patients with frontal lobe tumors have a better prognosis than those with temporal or parietal lobe tumors.22 A recent study of patients with glioblastoma reported longer survival in patients with MGMT gene promoter hypermethylation.39
Hospice care should be considered in patients who are not candidates for surgery or chemotherapy, in patients with deteriorating neurologic deficits despite therapy or tumor recurrence, and in patients with a poor performance status. Primary care physicians in conjunction with the neuro-oncologist and surgeon can help patients and families make decisions about hospice care. For a list of hospice care resources, go to https://www.aafp.org/afp/20070101/56.html.