
Am Fam Physician. 2008;78(2):244-246
Author disclosure: Nothing to disclose.
Clinical Question
In the primary care setting, what is the best way to identify patients with depression?
Evidence Summary
Although depression is common in the primary care setting, accurate identification of these patients is challenging. A variety of screening instruments for depression have been developed and validated. They range from relatively lengthy and complex instruments, such as the 21-item Beck Depression Inventory,1 to brief one-or two-item instruments.2 This article includes the brief screening instruments that are most widely used in the primary care setting.
Some instruments are well validated and accurate, but too long for routine use in the primary care setting, especially as screening tools. They are more appropriate in the research setting or to monitor patient response to treatment, particularly by sub-specialists. These instruments include the Beck Depression Inventory, the Hamilton Depression Rating Scale (HAM-D),3 and the Zung self-rating depression scale.4
A study of 164 outpatients with depression showed that a six-item version of the HAM-D scale has comparable sensitivity to the longer version, but has somewhat lower specificity.5 Another validation study found comparable accuracy between the six- and 21-item scales in a group of patients with Parkinson's disease, as measured by the area under the receiver operating characteristic curve.6 However, the six-item scale has not been validated in larger or more general primary care populations.
The four-item Brief Case-Find for Depression instrument is short enough for use in the primary care setting, but has not been broadly validated.7 Similarly, the Hospital Anxiety and Depression Scale has been validated primarily in more specialized populations and has limited accuracy (80 percent sensitivity, 69 percent specificity) in the primary care setting.8 A recent comparison of the five-item World Health Organization Well-Being Index (WBI-5) and the nine-item Patient Health Questionnaire (PHQ-9; Figure 1) found that the WBI-5 was slightly more sensitive, but significantly less specific than the PHQ-9.9
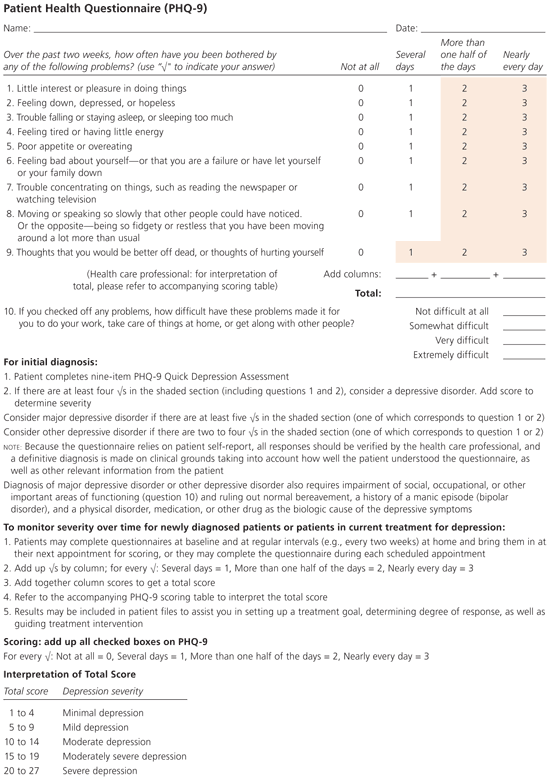
The most widely used and best-validated instruments in the primary care setting are the PHQ-9 and its two-item version, the PHQ-2 (Table 1). A meta-analysis of 14 studies found that the PHQ-9 is 81 percent sensitive and 92 percent specific for major depressive disorder in the primary care setting.10 Given a 10 percent pretest probability, a patient with a positive screen result has a 53 percent posttest probability of depression, and a patient with a negative result only has a 2.2 percent probability. In a higher-risk patient with a 30 percent pre-test probability, the posttest probabilities of depression are 81 percent with a positive screen result and 8.1 percent with a negative result. The PHQ-9 had variable sensitivity in more specialized populations, ranging from 54 percent in a cardiology service to 92 percent in a dialysis service; specificity was stable across settings.10
| During the past month: | |
| Have you often been bothered by feeling down, depressed, or hopeless? | |
| Have you often been bothered by little interest or pleasure in doing things? | |
The PHQ-2 has been evaluated in two large studies. The first, which included 1,419 outpatients, found the instrument to be 87 percent sensitive and 78 percent specific for major depressive disorder.11 The second, which included 6,000 primary care and obstetrics/gynecology outpatients, found it to be 83 percent sensitive and 92 percent specific.12 Thus, the two-question version is similar in accuracy to the longer version, but does not allow for the evaluation of severity or impact on daily activities.
A single-item screening question (Have you felt depressed or sad much of the time in the past year?) was evaluated in a study of outpatients at a Veterans Affairs hospital, but was shown to be less sensitive than the PHQ-2.2 The one-item version of the Yale-Brown Obsessive Compulsive Scale (“Do you often feel sad or depressed?”), which is similar to the PHQ-2, was 86 percent sensitive and 84 percent specific in a series of 122 patients hospitalized with stroke,13 and was 65 percent sensitive and 87 percent specific in a series of 120 patients with multiple sclerosis.14 There are no large validation studies in the primary care setting.
Ultimately, the PHQ-2 is the best brief screening instrument for use during a routine intake or annual physical examination survey. In patients with a positive screen result, the PHQ-9 or HAM-D instrument should be performed to confirm the diagnosis and assess severity. These scales can then be used prospectively to monitor response to therapy.