
Am Fam Physician. 2008;78(7):853-859
Patient information: See related handout on multiple myeloma, written by the authors of this article.
Author disclosure: Nothing to disclose.
Multiple myeloma, the most common bone malignancy, is occurring with increasing frequency in older persons. Typical symptoms are bone pain, malaise, anemia, renal insufficiency, and hypercalcemia. Incidental discovery on comprehensive laboratory panels is common. The disease is diagnosed with serum or urine protein electrophoresis or immunofixation and bone marrow aspirate analysis. Skeletal radiographs are important in staging multiple myeloma and revealing lytic lesions, vertebral compression fractures, and osteoporosis. Magnetic resonance imaging and positron emission tomography or computed tomography are emerging as useful tools in the evaluation of patients with myeloma; magnetic resonance imaging is preferred for evaluating acute spinal compression. Nuclear bone scans and dual energy x-ray absorptiometry have no role in the diagnosis and staging of myeloma. The differential diagnosis of monoclonal gammopathies includes monoclonal gammopathy of uncertain significance, smoldering (asymptomatic) and symptomatic multiple myeloma, amyloidosis, B-cell non-Hodgkin lymphoma, Waldenström macroglobulinemia, and rare plasma cell leukemia and heavy chain diseases. Patients with monoclonal gammopathy of uncertain significance or smoldering multiple myeloma should be followed closely, but not treated. Symptomatic multiple myeloma is treated with chemotherapy followed by autologous stem cell transplantation, if possible. Melphalan, prednisolone, dexamethasone, vincristine, doxorubicin, bortezomib, and thalidomide and its analogue lenalidomide have been used successfully. It is important that family physicians recognize and appropriately treat multiple myeloma complications. Bone pain is treated with opiates, bisphosphonates, radiotherapy, vertebroplasty, or kyphoplasty; nephrotoxic nonsteroidal anti-inflammatory drugs should be avoided. Hypercalcemia is treated with isotonic saline infusions, steroids, furosemide, or bisphosphonates. Because of susceptibility to infections, patients require broad-spectrum antibiotics for febrile illness and immunization against influenza, pneumococcus, and Haemophilus influenzae B. Five-year survival rates approach 33 percent, and the median survival rate is 33 months.
Multiple myeloma is the most common primary bone malignancy. More than 50,000 persons in the United States are currently diagnosed with multiple myeloma, and 16,000 are diagnosed annually.1 As the population ages, it is important for family physicians to diagnose myeloma and recognize its many complications.
Epidemiology
The median age at diagnosis of multiple myeloma is 70 years, and the occurrence increases with age. Age-adjusted rates of the disease are 6.9 per 100,000 men compared with 4.5 per 100,000 women; the rate is nearly twice as high in black persons than in the white population. Environmental factors likely interact with underlying genetic factors to increase the risk of multiple myeloma. Exposure to ionizing radiation, farming pesticides, or possibly petrochemicals also increases the risk. There is an increased incidence of multiple myeloma in persons with rheumatoid arthritis or obesity (body mass index of more than 30 kg per m2).2 However, no clear risk factor can be identified in most patients with this disease.
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| Serum and urine protein electrophoresis and immunofixation should be obtained for the diagnosis of multiple myeloma. | C | 6, 10, 11 |
| Nuclear bone scans and dual energy x-ray absorptiometry have no role in the diagnosis of multiple myeloma. | C | 6 |
| Smoldering (asymptomatic) multiple myeloma should not be treated. | A | 16 |
| Monitor for multiple myeloma symptoms and M protein (serum and urine) every six to 12 months in patients with monoclonal gammopathy of undetermined significance, and every three to four months in patients with smoldering multiple myeloma. | C | 3, 6, 16 |
| Avoid using nonsteroidal anti-inflammatory drugs when treating pain in patients with multiple myeloma. | C | 6 |
| Bisphosphonates should be prescribed for all patients with symptomatic multiple myeloma. | A | 6, 21 |
| Patients with multiple myeloma should receive influenza, pneumococcus, and Haemophilus influenzae B vaccinations. | B | 6, 29 |
Monoclonal gammopathy of undetermined significance (MGUS; a premalignant disorder in which a clone of plasma cells produces a monoclonal paraprotein that does not cause end-organ damage) is present in 2 percent of persons older than 50 years, and the risk of progressing to multiple myeloma is 1 percent each year.3 Several familial aggregations have been observed with an autosomal dominant pattern, increasing the risk by two- to fourfold.4
Pathophysiology
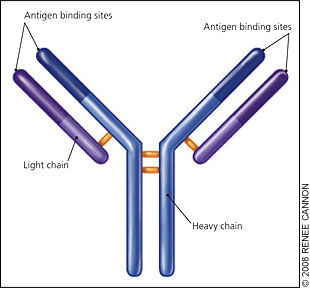
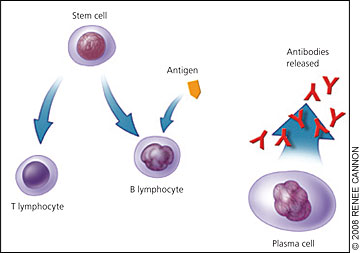
Immunoglobulin (Ig) molecules contain two linked heavy chains, with one light chain attached to each (Figure 1). Normally, plasma cells produce immunoglobulins to fight infection (Figure 2). However, monoclonal myeloma plasma cells proliferate and overproduce M protein (abnormal IgG, IgM, or IgA, or rarely IgE or IgD). Multiple myeloma cells also produce abnormal light chain proteins (κ or λ), cytokines that stimulate osteoclasts and suppress osteoblasts, and angiogenesis factors that promote new blood vessel formation. Therefore, the multiple myeloma process leads to an excessive M protein level, which causes hyperviscosity; light chain proteins that cause end-organ damage, especially in the kidneys; and invasive bone lesions that cause bone pain, osteoporosis, and hypercalcemia. Bone marrow invasion leads to anemia, and immunologic alterations contribute to recurrent infections.
Most new multiple myeloma cases are believed to arise de novo, although up to 20 percent evolve from MGUS.5 It is not completely understood how MGUS develops into multiple myeloma. An elevated M protein level (1.5 g per dL [15 g per L] or greater), non-IgG MGUS, and an abnormal free light chain ratio increase the risk of multiple myeloma to 58 percent over 20 years, if all three risk factors are present.3 Patients with MGUS should be monitored with laboratory tests every six to 12 months.3,6
Clinical Presentation
Many patients with multiple myeloma initially present with unexplained backache or bone pain. The long bones, ribs, skull, and pelvis are also commonly involved, and most patients have multiple lytic skeletal lesions. Pathologic fracture is the presenting symptom in 26 to 34 percent of patients.5,7 Vertebral compression fractures can lead to weakness and paresthesias in the lower extremities. Carpal tunnel syndrome is the most common peripheral neuropathy in patients with multiple myeloma. Anorexia, nausea, somnolence, and polydipsia are common symptoms of hypercalcemia. Weakness and malaise are usually associated with multiple myeloma anemia. Impaired antibodies and leukopenia cause recurrent infections, usually from encapsulated organisms (pneumonia is the most common infection). Weight loss occurs in less than one fourth of patients, and fever is rare at presentation. Table 1 lists the incidence of presenting symptoms in patients with multiple myeloma.5,7
| Symptom | Incidence (%) |
|---|---|
| Bone pain (especially back pain) | 58 |
| Fatigue (typically caused by anemia) | 32 |
| Pathologic fracture | 26 to 34 |
| Weight loss | 24 |
| Paresthesias | 5 |
| Fever | 0.7 |
| None (asymptomatic) | 34 |
It is important to note that 34 percent of patients are asymptomatic at presentation with incidental abnormalities on total protein, creatinine, calcium, or hemoglobin laboratory panels.5,7 Myeloma-related organ and tissue impairments include hypercalcemia: serum calcium level greater than 11 mg per dL (2.75 mmol per L); renal insufficiency: serum creatinine level greater than 2 mg per dL (180 μmol per L); anemia: hemoglobin level less than 10 g per dL (100 g per L); bone lesions: lytic lesions, compression fractures, or osteoporosis; and other impairments: symptomatic hyperviscosity, amyloidosis, or more than two bacterial infections within 12 months.6 These myeloma-related impairments are best remembered using the CRAB (calcium, renal, anemia, bone) mnemonic. Rare presentations include soft tissue or solitary bone masses (plasmacytomas), hyperviscosity-induced arterial infarctions or venous thrombosis, and concomitant amyloidosis with gastrointestinal symptoms, peripheral neuropathy, or cardiomegaly.8
Diagnosis
Bone or back pain and fatigue lasting more than two to four weeks in an older person, despite symptomatic treatment, should prompt further evaluation for multiple myeloma and several other conditions. Vitamin D deficiency, hyperparathyroidism, polymyalgia rheumatica, and bone metastasis must be considered in these patients. A complete blood count, erythrocyte sedimentation rate, chemistry panel, serum and urine protein electrophoresis, radiography, and vitamin D levels can assist in the differential diagnosis.
The diagnostic criteria for smoldering (asymptomatic) multiple myeloma is a serum M protein level of 3 g per dL (30 g per L) or more, 10 percent or more bone marrow plasma cells, and no related organ or tissue impairment (i.e., no end-organ damage, including bone lesions) or symptoms. The diagnostic criteria for symptomatic multiple myeloma is M protein (serum or urine), bone marrow clonal plasma cells or plasmacytoma, and myeloma-related organ or tissue impairment.9 Patients with symptoms suggestive of multiple myeloma (e.g., unexplained weakness, fatigue, back pain, proteinuria, anemia, renal insufficiency, recurrent infections, peripheral neuropathy) should be screened with serum and urine protein electrophoresis.
Serum and urine protein immunofixation are also recommended because immunofixation can be diagnostic even with a normal or nonspecific protein electrophoretic pattern.6,10,11 Urine dipstick tests are insensitive for Bence Jones protein, and urine protein electrophoresis and immunofixation are recommended in all patients with plasma cell dyscrasias.10 Bone marrow aspirate analysis should be performed in patients with abnormal serum or urine proteins and may require multiple samples because findings may be focal. Table 2 presents diagnostic abnormalities associated with multiple myeloma.5,10
| Abnormality | Percentage of patients with the abnormality | |
|---|---|---|
| M protein (serum or urine) | 97 | |
| Serum M protein | ||
| Electrophoresis | 82 | |
| Immunofixation | 93 | |
| Urine M protein | 75 | |
| 10 percent or more bone marrow plasma cells | 90 | |
| Bone lesions on radiography (lytic lesions, | 75 | |
| 66 percent of patients; fractures, | ||
| 26 percent; osteoporosis, 23 percent; blastic/sclerotic lesions, 0.5 percent) | ||
| Hemoglobin level of 12 g per dL (120 g per L) or less | 65 | |
| Serum creatinine level of 2 mg per dL (180 μmol per L) or greater | 23 | |
| Serum calcium level of 11 mg per dL (2.75 mmol per L) or greater | 13 | |
Most patients with symptomatic multiple myeloma have increased M protein levels (more than 3 g per dL) on serum protein electrophoresis. However, up to 20 percent of patients have a minimally elevated (less than 1 g per dL [10 g per L]) or normal serum γ-globulin level; the latter group has elevated urine excretion of light chains (κ or λ), which is often called Bence Jones myeloma. The type and amount of urine monoclonal light chains are typically measured with urine protein immunofixation. There is an even smaller subgroup (3 percent of multiple myeloma cases) with undetectable serum or urine M proteins on electrophoresis or immunofixation. This subgroup, called nonsecretory multiple myeloma, is diagnosed in patients with an abnormal serum free light chain ratio.12 Table 3 presents the differential diagnosis of monoclonal gammopathies.1–3,5,6,9,10,12
| Monoclonal gammopathy | Incidence | Serum findings | Bone marrow findings | Clinical clues |
|---|---|---|---|---|
| MGUS3 | 1 to 2 per 100 adults older than 50 years | M protein level of less than 3 g per dL (30 g per L) | Less than 10 percent plasma cells | Absence of myeloma-related organ and tissue impairment |
| Smoldering (asymptomatic) multiple myeloma5,10 | 5 to 7 per 1,000,000 | M protein level of 3 g per dL or greater (IgG, IgA, IgM, IgD, or free light chains) | 10 percent or more plasma cells | Absence of myeloma-related organ and tissue impairment |
| Symptomatic multiple myeloma6,10 | 5 to 7 per 100,000 | M proteins (40 percent of patients with multiple myeloma have a level less than 3 g per dL) | Plasma cells (5 percent of patients with multiple myeloma have fewer than 10 percent plasma cells) | Presence of at least one myeloma-related organ and tissue impairment |
| Waldenström macroglobulinemia10 | 7 to 10 per 1,000,000 | IgM | Biopsy findings are hypercellular with lymphocytes, plasma cells, and lymphoplasmacytoid cells | Epistaxis; vision, retinal, or neurologic problems |
| Amyloidosis2,10,12 | 5 to 13 per 1,000,000 | Ig light chains | Less than 10 percent plasma cells, Congo red amyloid bone marrow deposits (60 percent of patients) | Congestive heart failure, gastrointestinal symptoms, peripheral neuropathy |
| B-cell non-Hodgkin lymphoma1 | 19 per 100,000 adults | May have elevated M protein levels | Variable abnormal lymphocytes | Lymphadenopathy, fever, pruritus |
| Plasmacytoma5,10 | Rare | Extramedullary, IgA M protein | Solitary bone or soft tissue plasmacytoma shows plasma cells in the tumor; otherwise, there is no evidence of multiple myeloma in the bone marrow | Bone pain (spine or long bone), extramedullary (80 percent of cases are located in the upper respiratory tract) |
| Plasma cell leukemia10 | Rare | Low M protein levels, but more than 20 percent plasma cells in peripheral blood smear | More than 10 percent plasma cells (occurs de novo or with known multiple myeloma) | Lymphadenopathy, hepatosplenomegaly |
| Heavy chain diseases10 | Very rare | Incomplete heavy chains without light chains | Variable lymphocytes, plasma cells, lymphoplasmacytoid cells | Variable, depending on disease type (γ, α, or μ); autoimmune disease, malabsorption, lymphadenopathy, uvula or palatal edema |
Multiple myeloma anemia is typically normochromic and normocytic, although macrocytosis with vitamin B12 deficiency has been reported.13 Only 10 to 15 percent of patients have thrombocytopenia, and even fewer have leukocytosis—both conditions reflect severe plasma cell infiltration of the bone marrow. A complete blood count showing circulating plasma cells is uncommon, unless the disease is advanced. Sedimentation rates are typically greater than 50 mm per hour, although patients with Bence Jones myeloma often have values of less than 20 mm per hour.14
There is no standard imaging work-up for multiple myeloma, but a skeletal radiograph survey is generally recommended.6 Because radiography can take time, many institutions use whole-body radiographs with C-arm instruments designed for five-minute trauma scans. Lytic lesions are evident on radiography when 50 percent or more of trabecular bone is lost. The lesions appear on radiography even after successful treatment of multiple myeloma. Magnetic resonance imaging (MRI) is the preferred technique for suspected spinal compression or soft-tissue plasmacytomas. Computed tomography (CT) alone is more sensitive than plain radiography for small long bone lesions and can differentiate malignant from benign vertebral compression fractures in patients who are not MRI candidates. However, CT is not typically recommended for initial skeletal surveying. Although positron emission tomography with CT is not the standard of care, it is being used for staging and follow-up. Dual energy x-ray absorptiometry has no role in diagnosing multiple myeloma, and nuclear bone scans are not helpful because of the lack of osteoblastic activity.6
Several additional biochemical and genetic markers have been shown to correlate with prognosis in patients with multiple myeloma, but they should not be used for diagnosis. The β2-microglobulin level reflects tumor burden and renal impairment and, along with the serum albumin level, forms the basis of the International Staging System for multiple myeloma (Table 4).15 Interleukin-6, C-reactive protein, and plasma cell antigen (e.g., CD38, CD138) measurements, with DNA ploidy and cell cycle analysis, are increasingly being used to predict response to therapy.14
| Stage | Criteria | Percentage of patients in this stage | Median survival (months) |
|---|---|---|---|
| I | Serum β2-microglobulin level less than 3.5 mg per L (297 nmol per L) | 28 | 62 |
| Serum albumin level of 3.5 g per dL (35 g per L) or greater | |||
| II | Not stage I or III | 33 | 45 |
| III | Serum β2-microglobulin level of 5.5 mg per L (466 nmol per L) or greater | 39 | 29 |
Treatment
Although some patients with multiple myeloma do not require treatment, oncology referral is recommended in all patients. Patients with smoldering (asymptomatic) multiple myeloma should not undergo treatment.16 Earlier treatment has no effect on mortality and may increase the risk of acute leukemia.3,17 Three prognostic groups (with a mean time to progression of 27, 93, or 228 months) can be identified based on the percentage of bone marrow plasma cells and the amount of monoclonal protein. Patients with smoldering multiple myeloma should receive close follow-up with laboratory tests every three to four months.6,16
Autologous stem cell transplantation (ASCT) is the standard treatment for patients with symptomatic multiple myeloma who are younger than 65 years, and for older patients who are physically able to undergo the treatment. Patients who receive ASCT with high-dose induction chemotherapy have a median survival of 68 months.18,19 Induction protocols often use vincristine [Vincasar], doxorubicin [Adriamycin], and dexamethasone or dexamethasone and thalidomide (Thalomid) before ASCT to prevent myelosuppression from affecting the stem cell collection.11,15,19 Patients who are not candidates for ASCT generally receive melphalan (Alkeran) and prednisolone (Prelone) with or without thalidomide for initial therapy.11
Patients may have remission with a second course of the initial treatment; however, relapse is common with multiple myeloma. The proteasome inhibitor bortezomib (Velcade) and thalidomide and its analogue lenalidomide (Revlimid) have been shown to be beneficial; clinical trials are ongoing.6,11 Family physicians should be aware of the adverse effects of thalidomide (e.g., somnolence, neuropathy, deep venous thrombosis). Thalidomide neuropathy is not always reversible and may require discontinuation of therapy.20 With newer therapies, the overall five-year survival rate in patients with new multiple myeloma now approaches 33 percent, and the median survival is 33 months.5,11
Complications of Multiple Myeloma
Family physicians should be aware of myeloma complications (most notably, pain, bone disease, hypercalcemia, anemia, renal disease, and infection) and of treatment options. Pain control should be assessed at every office visit. Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided because of renal adverse effects.6 Opiates are the preferred therapies for pain control.
A Cochrane review showed that bisphosphonates decrease vertebral fractures and pain, but not mortality.21 Patients with symptomatic disease should receive bisphosphonates indefinitely, even in the palliative care phase. The intravenous bisphosphonates used in patients with multiple myeloma are pamidronate (Aredia), 90 mg every four weeks, and zoledronic acid (Zometa), 4 mg every four weeks.22 Oral bisphosphonates have not been proven effective. Osteonecrosis of the jaw is an unusual, painful adverse effect of bisphosphonate therapy and typically occurs after dental extraction.22 Neurosurgery and orthopedic referral for persistent vertebral pain may be beneficial. Effective surgical treatments are percutaneous vertebroplasty (i.e., injection of bone cement into the vertebra) and kyphoplasty (re-expanding the collapsed vertebra with a balloon before injecting methyl methacrylate).11,23 Single-fraction radiotherapy is effective in relieving metastatic bone pain, although retreatment and pathologic fracture rates are higher compared with multifraction radiotherapy.24 Spinal cord compression is treated with radiotherapy, although surgical decompression is performed for spinal instability. Long bone fractures require stabilization and subsequent pain control with radiotherapy.6
Renal insufficiency is common with multiple myeloma. Therapy focuses on identifying and correcting reversible causes, such as hypercalcemia, volume depletion, or drug-induced nephrotoxicity (especially with NSAIDs or contrast media). The most common cause of renal disease is cast nephropathy (myeloma kidney). Dialysis is performed when indicated, although the benefit of plasma exchange is debatable.26 Plasmapheresis can be used to treat hyperviscosity syndrome with arterial or venous thrombosis.
Anemia is common at onset of disease and with progression. Anemia generally responds to myeloma treatment. Symptomatic anemia is managed with transfusions and erythropoietin therapy, which improves hemoglobin levels and lowers transfusion rates in patients with multiple myeloma,27 although the effect of erythropoietin on quality of life may be overstated.28 Other causes of anemia, including bleeding, iron deficiency, vitamin B12 or folate deficiency, and hemolysis, should be considered.