
Am Fam Physician. 2009;79(1):29-36
A more recent article on type 2 diabetes mellitus is available.
Patient information: See related handout on lifestyle changes to manage type 2 diabetes, written by the authors of this article.
Author disclosure: Nothing to disclose.
Evidence-based guidelines for the treatment of type 2 diabetes mellitus focus on three areas: intensive lifestyle intervention that includes at least 150 minutes per week of physical activity, weight loss with an initial goal of 7 percent of baseline weight, and a low-fat, reduced-calorie diet; aggressive management of cardiovascular risk factors (i.e., hypertension, dyslipidemia, and microalbuminuria) with the use of aspirin, statins, and angiotensin-converting enzyme inhibitors; and normalization of blood glucose levels (hemoglobin A1C level less than 7 percent). Insulin resistance, decreased insulin secretion, and increased hepatic glucose output are the hallmarks of type 2 diabetes, and each class of medication targets one or more of these defects. Metformin, which decreases hepatic glucose output and sensitizes peripheral tissues to insulin, has been shown to decrease mortality rates in patients with type 2 diabetes and is considered a first-line agent. Other medications include sulfonylureas and nonsulfonylurea secretagogues, alpha glucosidase inhibitors, and thiazolidinediones. Insulin can be used acutely in patients newly diagnosed with type 2 diabetes to normalize blood glucose, or it can be added to a regimen of oral medication to improve glycemic control. Except in patients taking multiple insulin injections, home monitoring of blood glucose levels has questionable utility, especially in relatively well-controlled patients. Its use should be tailored to the needs of the individual patient.
Type 2 diabetes mellitus, the sixth leading cause of death in the United States, is directly responsible for more than 73,000 deaths annually and is a contributing factor in more than 220,000 deaths.1 It is the leading cause of kidney failure and new cases of blindness in adults,1 and it is a significant cause of lost workforce productivity.2 More than 20 million Americans have diabetes; 6 million of these are undiagnosed.1 Ethnic and racial minorities are disproportionately affected.1 Derangement of glucose homeostasis and the eventual development of diabetes is a multifactorial process involving genetics, ethnic and racial heritage, and environmental factors. Although the precise interplay of these factors is not yet fully understood, long-term trials have provided evidence to support aggressive efforts to prevent and manage this disease (Table 1).3–6
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| Patients with impaired glucose tolerance should be provided with counseling and instruction for weight loss and physical activity. | A | 6, 8 |
| Metformin (Glucophage) is the only medication proven to reduce mortality rates in patients with type 2 diabetes. | A | 5 |
| Acarbose (Precose) seems to reduce the risk of cardiovascular disease events. | B | 13, 1719–21 |
| When adding insulin to a regimen of oral medication, oral agents should be continued initially. Long-acting insulin should be used initially, typically at a dosage of 10 units per day or 0.17 to 0.5 units per kg per day, and titrated in increments of two units approximately every three days. | C | 14, 24 |
| Trial | Years (average duration) | Design | Participants | Intervention | Results | Clinical contributions | ||
|---|---|---|---|---|---|---|---|---|
| UKPDS3,4 | 1977 to 1991 (10 years) | Multicenter randomized controlled trial | 3,867 Newly diagnosed with type 2 diabetes; average age, 54 years | Sulfonylureas, insulin, or dietary intervention | Treatment reduced risk of microvascular end points (e.g., need for retinal photocoagulation) by 25 percent (95% CI, 7 to 40 percent) Reduced mortality rates with blood pressure and lipid control | Blood pressure and lipid control greatly reduce cardiovascular mortality rates in patients with diabetes Tight blood glucose control reduces retinal microvascular complications The highest annual incidence of major hypoglycemic events was 2.3 percent and occurred in patients on insulin therapy | ||
| UKPDS (second arm within the larger trial)5 | 1977 to 1991 (10.7 years) | Randomized embedded trial | 753 Newly diagnosed with type 2 diabetes; BMI at randomization > 120 percent of ideal | Metformin (Glucophage) or dietary intervention Secondary analysis compared metformin with insulin and sulfonylureas Another secondary analysis compared the addition of metformin to sulfonylureas when participants failed treatment with sulfonylureas | 36 percent reduction in all-cause mortality (P = .011) and 42 percent reduction in diabetes-related death (P = .017) with use of metformin compared with conventional therapy | Metformin should be the drug of choice in patients with type 2 diabetes, particularly in obese patients | ||
| Diabetes Prevention Program6 | 1996 to 1999 (2.8 years) | Multicenter randomized controlled trial | 3,234 At least 25 years of age (mean, 51 years) with BMI ≥ 24 kg per m2 (mean, 34 kg per m2), fasting glucose level of 95 to 125 mg per dL (5.30 to 6.95 mmol per L), and glucose level of 140 to 199 mg per dL (7.75 to 11.05 mmol per L) two hours post-glucose load | Metformin, placebo, or intensive lifestyle intervention, which included 150 minutes of weekly exercise and a goal of 7 percent weight loss | Average weight loss: | Incidence of diabetes: | ||
| Placebo: 0.1 kg | Placebo: 11.0 cases per 100 person-years | |||||||
| Metformin: 2.1 kg | Metformin: 7.8 cases per 100 person-years | |||||||
| Lifestyle: 5.6 kg | Lifestyle: 4.8 cases per 100 person-years | |||||||
| Reduction in daily energy intake (in kcals): | Number needed to treat to prevent one new case of diabetes in three years: | |||||||
| Placebo: 249 ± 27 | ||||||||
| Metformin: 296 ± 23 | Metformin: 13.9 | |||||||
| Lifestyle: 450 ± 26 | Lifestyle: 6.9 | |||||||
Management of Type 2 Diabetes
Evidence-based guidelines for the comprehensive management of diabetes focus primarily on lifestyle changes, management of cardiovascular disease risk factors, and management of blood glucose levels.7
LIFESTYLE CHANGES
Lifestyle modification can help patients lose weight and reduces the incidence of type 2 diabetes in at-risk patients.8 One large study compared usual care with an intensive lifestyle intervention.6 Although only 38 percent of participants achieved and maintained the weight loss goal of 7 percent of baseline body weight, the incidence of type 2 diabetes was reduced by 58 percent. To prevent one new case of diabetes in three years, 6.9 persons would need to undergo intensive lifestyle intervention.6 Lifestyle changes were much more effective than metformin (Glucophage) therapy. In a review of 14 trials testing exercise interventions in participants with type 2 diabetes, hemoglobin A1C levels were reduced by 0.6 percent, and triglyceride levels and visceral adiposity were decreased independent of weight loss.9 These results underscore the importance of reinforcing lifestyle goals with every patient at every visit, even if weight loss falls short of expectations.
MANAGEMENT OF CARDIOVASCULAR DISEASE RISK FACTORS
Multifactorial interventions to manage cardiovascular disease risk factors (i.e., blood pressure, cholesterol, microalbuminuria) in patients with type 2 diabetes have been shown in well-designed clinical trials to decrease mortality rates.10 Daily low-dose aspirin is recommended for patients with type 2 diabetes and coronary artery disease (CAD), those older than 40 years, and those who have additional risk factors for cardio-vascular disease (e.g., family history of cardiovascular disease, hypertension, smoking, dyslipidemia, albuminuria).7 Statins are recommended for patients with type 2 diabetes and CAD, and for patients with diabetes without CAD who are older than 40 years and have one other cardiovascular disease risk factor.7 Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are mainstays of treatment for patients with micro- or macroalbuminuria.7
MANAGEMENT OF BLOOD GLUCOSE LEVELS
Insulin resistance, decreased insulin secretion, and increased hepatic glucose output are the hallmarks of type 2 diabetes. Medications target one or more of these defects (Table 2).11–13 Average absolute reductions in A1C for each class of medication range from 0.5 to 1.0 percent for exenatide (Byetta), pramlintide (Symlin), and alpha-glucosidase inhibitors to 1 to 2.5 percent for sulfonylureas and metformin.14 Reviews have reported that mono-therapy with any oral hypoglycemic agent is superior to dietary management or placebo in reducing A1C values, but the studies are so heterogeneous that the expected A1C reduction attributed to any class of medication should be interpreted with caution.15,16 For example, six trials that evaluated sulfonylureas for an average of 16 weeks reported mean A1C reductions of 1.8 percent (range, 1 to 2.5 percent),15 whereas the 10-year United Kingdom Prospective Diabetes Study reported an A1C reduction of 0.9 percent with sulfonylureas.3 This suggests that short-term studies may not accurately reflect long-term results. It is also critical to remember that the goal of treatment is not only to reduce A1C levels, but also to prevent premature mortality and morbidity. Not all agents have been proven to achieve this goal.
| Class | Drug | Potential side effects | Contraindications | Comments | Relative cost* |
|---|---|---|---|---|---|
| Alpha glucosidase inhibitors | Acarbose (Precose) Miglitol (Glyset) | Flatulence; abdominal pain; diarrhea | — | To reverse hypoglycemia (usually only in setting of combination therapy), treat with oral glucose, not sucrose | $$ |
| Amylin analogues | Pramlintide (Symlin) | Nausea and vomiting; anorexia; headache | Gastroparesis; lack of awareness of hypoglycemia; A1C > 9 percent | Severe hypoglycemia can occur, especially with coadministration of insulin; injectable medication; reduce insulin dose by 50 percent when initiating therapy | $$ |
| Biguanides | Metformin (Glucophage) | Nausea; diarrhea; flatulence | Renal insufficiency (discontinue if creatinine level ≥ 1.4 in women or ≥ 1.5 in men); conditions that predispose to acidosis (e.g., liver disease, hypoxemia, sepsis); discontinue during acute illness and before radiographic procedures requiring intravenous dye (may restart 48 hours after procedure if serum creatinine levels are unchanged) | Decreases circulating androgen levels and increases rates of ovulation in women with polycystic ovarian syndrome; modest weight loss may occur; pregnancy category B based on animal studies but not well-studied in pregnant women | $† |
| Incretin enhancers | Saxagliptin (Onglyza)‡ Sitagliptin (Januvia) | Nausea and vomiting | Adjust dosage in patients with renal impairment | — | NA |
| Incretin mimetics | Exenatide (Byetta) | Nausea and vomiting; diarrhea; dizziness | Not recommended in patients with severe renal disease (creatinine clearance < 30 mL per minute) | Injectable medication; modest weight loss may occur | $$$$ |
| Insulin secretagogues: sulfonylureas | Chlorpropamide (Diabinese)§ | Hypoglycemia; weight gain Glimepiride (Amaryl) Glipizide (Glucotrol) Glyburide (Micronase) Tolazamide (Tolinase)§ Tolbutamide (Orinase)§ | — | — | $† |
| Insulin secretagogues: nonsulfonylureas | Nateglinide (Starlix) | Hypoglycemia Repaglinide (Prandin) | — | Metabolized through CYP3A4 | $$$ |
| Thiazolidinediones | Pioglitazone (Actos) Rosiglitazone (Avandia) | Weight gain; fluid retention | Hepatic disease; alanine transaminase level > 2.5 times normal; pregnancy; congestive heart failure (New York Heart Association class III or IV); use with caution in patients with edema | Association between rosiglitazone and cardiovascular events12,13 | $$$$ |
Insulin Secretagogues
Sulfonuylurea insulin secretagogues (e.g., glipizide [Glucotrol], glimepiride [Amaryl]) and nonsulfonylurea insulin secretagogues (e.g. nateglinide [Starlix]) increase insulin secretion by closing potassium channels on the surface of pancreatic beta cells.11 Hypoglycemia can occur with any insulin secretagogue. Sulfonylureas can cause weight gain; this effect is less common with nonsulfonylurea secretagogues. A recent review concluded that cardiovascular disease events are neither increased nor decreased with the use of sulfonylureas.17 There is insufficient evidence to make any conclusions about the effects of nonsulfonylurea secretagogues on cardiovascular morbidity and mortality.17
Biguanides
Metformin decreases hepatic glucose output and, to a lesser extent, sensitizes peripheral tissues to insulin.11 A review representing more than 36,000 patient-years of metformin use found no increase in fatal or nonfatal lactic acidosis.18 However, current guidelines recommend that metformin should not be used in patients with chronic or acute renal insufficiency, and should be discontinued when creatinine levels reach 1.4 mg per dL (120 μmol per L) in women or 1.5 mg per dL (130 μmol per L) in men. Metformin has been shown to decrease progression from impaired glucose tolerance to type 2 diabetes.6 To prevent one new case in three years, 13.9 persons would have to be treated with metformin.6 It is the only hypoglycemic agent shown to reduce mortality rates in patients with type 2 diabetes.5
Thiazolidinediones
Thiazolidinediones increase insulin sensitivity in peripheral tissues and, to a lesser extent, decrease hepatic glucose production.11 These agents will not cause hypoglycemia when used as monotherapy. A recent review of 18 trials concluded that rosiglitazone (Avandia) is associated with an increased risk of myocardial infarction (MI) and death from cardiovascular causes.12 Another review of four trials concluded that the risk of MI and heart failure are significantly increased, but overall cardiovascular mortality rates are unaffected.13 The latter review was limited to trials with one or more years of follow-up, whereas the former review included trials with shorter follow-up periods. In a meta-analysis of 19 controlled trials, pioglitazone (Actos) was associated with a reduction in a composite end point of death, MI, and stroke.19 The incidence of serious heart failure was increased by 40 percent, but there was no change in cardiovascular disease mortality rates.
Alpha-Glucosidase Inhibitors
Alpha-glucosidase inhibitors act at the brush border in the small intestine, inactivating the enzyme that breaks down complex carbohydrates, slowing absorption, and flattening the postprandial glycemic curve.11 Acarbose (Precose) reduces the risk of cardiovascular disease events, including acute MI, in patients with impaired glucose tolerance or type 2 diabetes.17,20,21
Incretin Mimetics and Incretin Enhancers
Incretin hormones stimulate glucose-dependent insulin secretion, decrease glucagon secretion, slow gastric emptying, and decrease appetite.11 Exenatide lowers blood glucose levels and stimulates weight loss, perhaps by slowing gastric emptying and producing satiety.11,22 Sitagliptin (Januvia) has no effect on body weight.23 There are no data on the effects of these medications on cardiovascular events.17
Amylin Analogues
Pramlintide is an amylin analogue indicated for use in patients with type 1 diabetes; it is rarely used to manage type 2 diabetes.11 When pramlintide is initiated, the insulin dosage should be reduced by 50 percent to avoid potentially severe hypoglycemia. There is insufficient evidence to make conclusions about the effects of pramlintide on cardiovascular disease.17
Approach to the Patient
Algorithms for the management of blood glucose contain elements derived from large, well-designed clinical trials, but the algorithms themselves are compiled from expert opinion and have not been conclusively evaluated14,24 (Figure 1).7,14,24 The goal is to maintain blood glucose levels as close to normal as possible without risking significant hypoglycemia. The American Diabetes Association recommends an A1C goal of less than 7 percent.7 Glycemic control requires the patient to have cognitive, visual, and motor skills to monitor and manage blood glucose levels, and identifying and minimizing barriers for effective self-management is an important first step to setting individualized goals. There are no evidence-based recommendations for the frequency of home blood glucose monitoring except for patients administering multiple daily injections of insulin; several studies have questioned the usefulness of home monitoring.25,26 In patients with relatively well-controlled diabetes, home monitoring has not been associated with clinically significant improvements in A1C levels.25,26 Monitoring can be a useful tool in adjusting medications in the three-month intervals between A1C measurement, but it is also expensive and time-consuming, and it should be individualized to meet the needs of each patient.
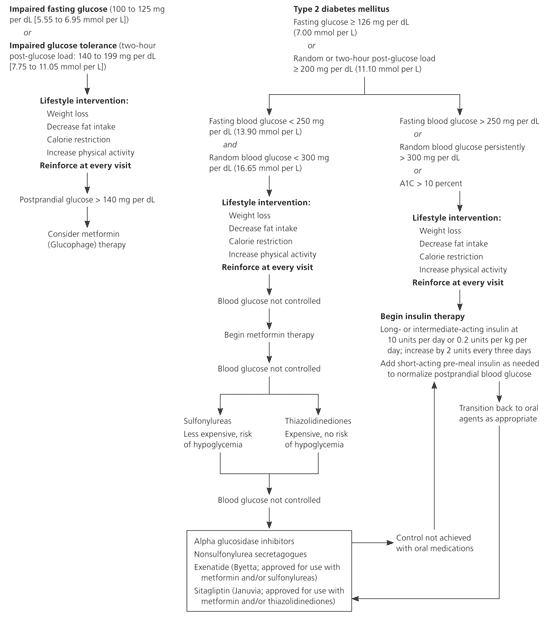
INITIAL MANAGEMENT
The first step in managing type 2 diabetes is to normalize fasting glucose levels, with weekly or monthly adjustments in the regimen.14 Metformin is a first-line consideration. Gastrointestinal symptoms associated with its use can be minimized by beginning with a low dose and titrating slowly. Additional agents include sulfonylureas, nonsulfonylurea secretagogues, thiazolidinediones, and alpha-glucosidase inhibitors. Any of these agents can be combined with another. Once fasting blood glucose approaches near-normal levels, postprandial glucose is addressed by increasing the dose of the current medications or by adding additional agents. Once maximal benefit is achieved from first-line medications, other agents, such as exenatide (approved for use with metformin or sulfonylureas) and sitagliptin (approved for use with metformin or thiazolidinediones), can be considered.
INITIATING INSULIN THERAPY
Less than 40 percent of patients with diabetes successfully achieve an A1C level of less than 7 percent.24 One reason for this is the reluctance of patients and physicians to start insulin therapy, perceiving it as a treatment failure. However, progressive failure of the beta cells often occurs even with proper diet, exercise, and oral medications, so patients should be counseled that insulin is simply another management tool. Although insulin is typically introduced when glucose control is no longer possible with oral agents, it can also be used when contraindications to oral medications exist. Newly diagnosed patients also can benefit from acute insulin use. Prolonged hyperglycemia can cause glucose toxicity, a potentially reversible impairment in glucose-stimulated insulin secretion. This can be corrected with aggressive insulin therapy, then oral medications can be added as insulin is tapered or discontinued. Expert opinion suggests that insulin therapy should be initiated if the fasting blood glucose level is consistently greater than 250 mg per dL (13.90 mmol per L), or if random testing shows levels greater than 300 mg per dL24 (16.65 mmol per L; Figure 17,14,24).
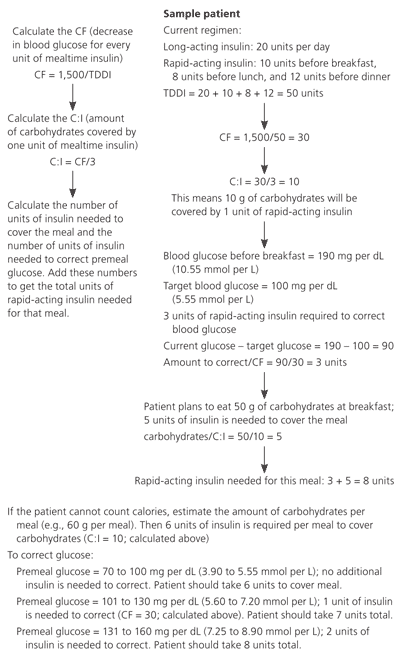
When adding insulin to an oral medication regimen, oral agents should initially be continued. Long-acting insulin should be used initially, typically at a dosage of 10 units per day or 0.17 to 0.5 units per kg per day, and titrated in increments of two units approximately every three days14,24 (Table 3). Rapid-acting or premixed preparations can be added if fasting blood glucose levels are persistently high or if A1C has plateaued at about 7.5 percent, which indicates that postprandial glucose levels are high. Adding more basal insulin in this setting usually will not help patients reach their target levels.24 Sliding-scale doses can be set by counting carbohydrate grams or by a preset scale (Figure 2). For the latter method, one suggested regimen is to give 90 percent of the basal dose of insulin in long-acting form and the remainder in rapid-acting form at the largest meal, then adjust the dose as necessary.24 Insulin is used almost exclusively in pregnancy because of the concern of teratogenicity with oral medications.
| Insulin preparation | Onset of action | Peak | Duration of action | Comments |
|---|---|---|---|---|
| Rapid-acting insulin | ||||
| Lispro (Humalog) | 5 to 15 minutes | 1 to 2 hours | 4 to 5 hours | — |
| Aspart (Novolog) | 5 to 15 minutes | 1 to 2 hours | 4 to 5 hours | — |
| Glulisine (Apidra) | 5 to 15 minutes | 1 to 2 hours | 4 to 5 hours | — |
| Regular (recombinant) (Humulin R) | 30 to 60 minutes | 2 to 4 hours | 8 to 10 hours | Inject 30 minutes before meal |
| Intermediate-acting insulin | ||||
| Isophane (NPH) (Humulin N) Long-acting insulin | 1 to 2 hours | 4 to 8 hours | 10 to 20 hours | — |
| Detemir (recombinant) (Levemir) | 1 to 2 hours | Relatively flat | 12 to 20 hours | Smoother curve than NPH; administered once or twice daily; available in pen form; can be kept without refrigeration for up to 42 days |
| Glargine (Lantus) | 1 to 2 hours | Relatively flat | 20 to 24 hours | Available in pen form |
| Mixed insulin | ||||
| Multiple preparations (e.g., Humulin 70/30) | 30 minutes | Dual peak | Up to 24 hours | Mixed insulin preparations may hinder tight glycemic control because the ratio of the two preparations cannot be altered |
CHILDREN AND OLDER ADULTS
As the prevalence of obesity in children has increased, type 2 diabetes has also become more common. Metformin is approved for use in children 10 years and older and sustained-release preparations are approved for use in persons 17 years and older who cannot maintain glycemic control with diet and exercise.7,27
The increased prevalence of comorbid conditions in older adults requires careful consideration of medications. Serum creatinine levels are not always a reliable predictor of renal insufficiency in the elderly, so metformin should be used with caution. The high prevalence of heart failure in this population limits the use of thiazolidinediones. Older patients are likely to benefit more from aggressive management of known cardiovascular disease risk factors such as hypertension than by tight glycemic control, which can increase symptomatic hypoglycemia.7