
Am Fam Physician. 2009;79(9):761-767
This is part I of a two-part article on asthma management guidelines. Part II will appear in a future issue of AFP.
Patient information: See related handout on asthma, written by the authors of this article.
Author disclosure: Nothing to disclose.
The Expert Panel Report 3 of the National Asthma Education and Prevention Program represents a major advance in the approach to asthma care by emphasizing the monitoring of clinically relevant aspects of care and the importance of planned primary care, and by providing patients practical tools for self-management. Treatment of asthma should be guided by a new system of classification that assesses severity at initial evaluation and control at all subsequent visits. Asthma severity is determined by current impairment (as evidenced by impact on day-to-day activities) and risk of future exacerbations (as evidenced by frequency of oral systemic corticosteroid use), and allows categorization of disease as intermittent, persistent-mild, persistent-moderate, and persistent-severe. Initial treatment is guided by the disease-severity category. The degree of control is also determined by the analysis of current impairment and future risk. Validated questionnaires can be used for following the impairment domain of control with patients whose asthma is categorized as “well controlled,” “not well controlled,” and “very poorly controlled.” Decisions about medication adjustment and planned follow-up are based on the category of disease control. Whereas a stepwise approach for asthma management continues to be recommended, the number of possible steps has increased.
The National Asthma Education and Prevention Program released its Expert Panel Report 3 (EPR-3) on Guidelines for the Diagnosis and Management of Asthma in 2007.1 Whereas previous versions of these guidelines focused on disease classification and stepwise care as methods for optimally managing patients with asthma,2 the latest update looks at this issue through a different, broader lens. This new, multidimensional approach allows family physicians and their health care team to subjectively evaluate individual patients in the context of their home, daily activities, and work and family environments—an approach that resonates with the New Model of family medicine described in the Future of Family Medicine Project.3 In this article, concepts of this new approach are discussed, and some tools for incorporating this approach into the care of individual patients are provided.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Every patient with asthma should be able to recognize symptoms that suggest inadequate asthma control. | C | 4, 5 |
| Validated questionnaires exist and are useful in following the impairment domain of control. | C | 6–9 |
| All patients, regardless of management step, should be given a prescription for a short-acting beta agonist and instructed in its appropriate use. | C | 14 |
| Inhaled corticosteroids improve asthma control more effectively in children and adults than any other single long-term controller medication. | A | 15, 16 |
| Written action plans detailing medications and environmental control strategies tailored for each patient are recommended for all patients with asthma. | B | 17–23 |
Evaluation
The paradigm on which the EPR-3 report is based focuses on two aspects of asthma evaluation (i.e., severity and control) in determining level of treatment, and two concepts (i.e., current impairment and future risk) in guiding treatment choice at each level of care.
SEVERITY AND CONTROL
Consistent throughout the previous guidelines has been the classification of asthma into subgroups based on severity, with treatment based on those subgroups. A patient presenting with previously undiagnosed asthma could readily be classified based on the objective and subjective criteria provided by the guidelines. However, patients with a preexisting asthma diagnosis who were being treated were more difficult to classify. Those with uncontrolled but treated disease were equally difficult to classify, given that they were likely in a higher classification than their current medication regimen suggested, and only step-up therapy would clarify the issue. Finally, a large subset of patients with asthma could be classified differently depending on their exposure at the time of classification. For example, disease severity could increase during allergy season or with other trigger exposures and decrease for a period after these exposures.
The updated guideline moves away from a rigid categorization of disease and recognizes that identifying disease severity alone does not lead to optimal asthma management. The key elements of assessment and monitoring are refined to include the separate but related concepts of severity, control, and responsiveness to treatment. Classifying severity is emphasized for initiating therapy; assessing control is emphasized for monitoring and adjusting therapy. Asthma control is now weighted equally with asthma severity in determining appropriate therapy, with the recognition that asthma severity can change over time and is most readily recognized by ongoing care of asthma.
IMPAIRMENT AND RISK
The EPR-3 report recommends that the assessment of severity and control be considered as two domains: impairment and risk. Impairment refers to the limitations in activity or the degree of symptoms on a day-to-day basis. For family physicians, this is the clinically relevant aspect of asthma care and is an essential component in adjusting asthma therapy. In addition, the new guidelines introduce “risk” as a second parameter that should be systematically monitored in patients with asthma. Risk assessment takes into consideration what the physician thinks will happen if the patient remains on his or her current medication regimen. It is based on the patient's severity and history of asthma, as well as what the probability of exacerbations will be for the next several months.
In identifying disease severity, an exacerbation requiring oral systemic corticosteroids is the marker of risk. In assessing asthma control, risk not only includes the history of exacerbations, but also incorporates objective measurements of lung function and treatment-related adverse events. Final decisions about severity or control classifications are guided by the most severe category in which any feature of impairment or risk occurs.
CLASSIFICATION OF ASTHMA SEVERITY
Ideally, asthma severity is determined before initiating therapy. The EPR-3 guideline classification divides asthma severity into four groups: intermittent, persistent-mild, persistent-moderate, and persistent-severe. “Mild-intermittent,” a classification in previous reports, has been eliminated. This term really only applies to mild disease, and not to patients with periods of moderate or severe exacerbation.
Classification of a patient's disease also depends on current impairment and future risk. Impairment is determined by patient symptoms and objective measurement of lung function. The guideline recommends that, at a minimum, assessments of the patient's symptoms include daytime symptoms, nighttime awakenings, frequency of short-acting beta agonist use for symptom relief, and inability to do (or difficulty with) normal activities because of symptoms (Table 11). Spirometry is recommended as a component of the determination of current impairment. As mentioned previously, future risk is categorized by the frequency of oral systemic corticosteroid use.
| Components of severity | Classification of asthma severity ≥ 12 years of age* | |||
|---|---|---|---|---|
| Intermittent | Persistent-mild | Persistent-moderate | Persistent-severe | |
| Impairment | ||||
| Symptoms | ≤ 2 days per week | > 2 days per week, but not daily | Daily | Throughout the day |
| Nighttime awakenings | ≤ 2 times per month | 3 to 4 times per month | > Once per week, but not nightly | Often 7 times per week |
| Short-acting beta agonist use for symptom control (not for prevention of exercise-induced bronchospasm) | ≤ 2 days per week | > 2 days per week, but not more than once per day | Daily | Several times per day |
| Interference with normal activity | None | Minor limitation | Some limitation | Extremely limited |
| Lung function | Normal FEV1 between exacerbations; FEV1 >80 percent of predicted; FEV1/FVC normal | FEV1 ≥ 80 percent of predicted; FEV1/FVC normal | FEV1 > 60 percent but < 80 percent of predicted; FEV1/FVC reduced 5 percent | FEV1 < 60 percent of predicted; FEV1/FVC reduced >5 percent |
| Risk | ||||
| Exacerbations requiring oral systemic corticosteroids | 0 to 1 per year† | ≥ 2 per year† | ≥ 2 per year† | ≥ 2 per year† |
| Consider severity and interval since last exacerbation; frequency and severity may fluctuate over time for patients in any severity category; relative annual risk of exacerbations may be related to FEV1 | ||||
CLASSIFICATION OF ASTHMA CONTROL
The guideline recommends that every patient with asthma be able to recognize symptoms that suggest inadequate asthma control.4,5 As with asthma severity, assessment of control is determined by current impairment and future risk. The symptoms and history used to determine current impairment are the same as those used to determine impairment in evaluating disease severity, namely daytime symptoms, nighttime awakenings, frequent use of short-acting beta agonists for symptom relief, and inability to do (or difficulty with) normal activities because of symptoms.
Several questionnaires have been validated for the evaluation of symptom control.6–9 The Asthma Therapy Assessment Questionnaire (http://www.asthmacontrolcheck.com/asthma_control/asthmacontrolcheck/consumer/index.jsp),10 the Asthma Control Questionnaire (http://aafa.org/pdfs/SWP%20final%20questionnaire.pdf),11 and the Asthma Control Test (http://www.asthmacontrol.com)12 provide validated control “scores” that can be used to categorize asthma into three control categories: well controlled, not well controlled, and very poorly controlled.
A final recommended determinant of current impairment is forced expiratory volume in one second (FEV1) or peak expiratory flow (PEF) rate, with 80 percent or more of predicted or personal best categorizing patients' asthma as well controlled, and less than 60 percent of predicted or personal best indicating very poor control. The available data suggest symptom monitoring and peak flow monitoring have similar benefits in determining asthma control.13 Long-term, daily peak flow monitoring can be useful, particularly in patients with moderate- to severe-persistent asthma. Regardless of which parameters are used, self-monitoring is important for the effective self-management of asthma.
Treatment Recommendations
INITIATING MEDICATION
A new diagnosis of asthma requires important decisions about the initiation of an optimal regimen of medication. Compared with previous guidelines, the EPR-3 report provides more targeted recommendations for initiation of pharmacologic therapy. Table 11 provides a guide for classifying asthma severity in older children and adults based on current impairment and anticipated risk, with the severity class based on the most severe category in which any of the components occur. Separate tables apply to patients younger than five years and those from five to 12 years of age, but the frameworks are similar.
Each severity class is assigned a recommended step for initiating pharmacologic therapy, with each step having preferred and alternative medication choices (Figure 11 ). Patients should be reevaluated two to six weeks after the introduction of medication, at which time asthma control is used to adjust medication. There is a preference for treating more aggressively to obtain rapid control, then stepping down to a maintenance regimen. All patients, regardless of step, should be given a prescription for a short-acting beta agonist and instructed in its use.14 Based on evidence of enhanced drug distribution and effectiveness, the EPR-3 guideline emphasizes the benefits of spacers for everyone using a metered-dose inhaler.
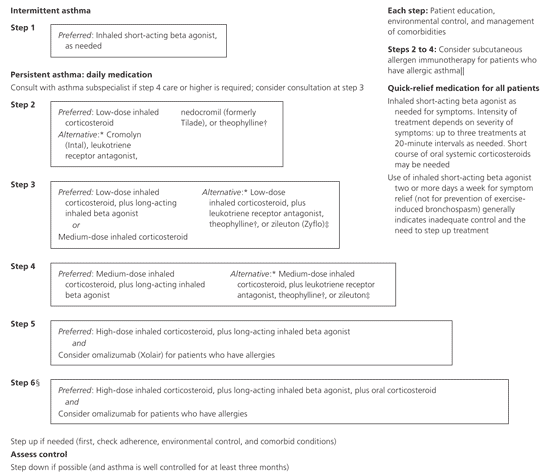
CONTROL AS A GUIDE TO MEDICATION ADJUSTMENT
After targeted, step-based initiation of pharmacologic therapy, the classification of asthma control is used to adjust medication, stepping up or down depending on the level of control. Patients whose asthma can be classified as “well controlled” can be maintained on their current medications and, if stable for at least three months, a step down in therapy can be considered (Figure 11 and Table 21). Patients whose asthma is classified as “not well controlled” on their initial therapy are advised to step up one step and be reevaluated in two to six weeks; for patients with very poorly controlled asthma, consider short-term oral systemic corticosteroid use and stepping up one or two steps, then reassessing in another two to four weeks.
| Components of control | Classification of asthma control (≥ 12 years of age)* | ||
|---|---|---|---|
| Well controlled | Not well controlled | Very poorly controlled | |
| Impairment | |||
| Symptoms | ≤ 2 days per week | > 2 days per week | Throughout the day |
| Nighttime awakenings | ≤ 2 times per month | 1 to 3 times per week | ≥ 4 times per week |
| Interference with normal activity | None | Some limitation | Extremely limited |
| Short-acting beta agonist use for symptom control (not for prevention of exercise-induced bronchospasm) | ≤ 2 days per week | > 2 days per week | Several times per day |
| FEV1 or peak flow | > 80 percent of predicted/personal best | 60 to 80 percent of predicted/personal best | < 60 percent of predicted/personal best |
| Validated questionnaires | |||
| ATAQ | 0 | 1 to 2 | 3 to 4 |
| ACQ | ≤ 0.75† | ≥ 1.5 | — |
| ACT | ≥ 20 | 16 to 19 | ≤ 15 |
| Risk | |||
| Exacerbations requiring oral systemic corticosteroids | 0 to 1 time per year‡ | ≥ 2 times per year‡ | ≥ 2 times per year‡ |
| Consider severity and interval since last exacerbation | |||
| Progressive loss of lung function | Evaluation requires long-term follow-up care | ||
| Treatment-related adverse effects | Medication adverse effects can vary in intensity from none to very troublesome and worrisome; the level of intensity does not correlate to specific levels of control, but should be considered in the overall assessment of risk | ||
| Recommended action for treatment (see Figure 1 for treatment steps) | Well controlled: Maintain current step; regular follow-up every one to six months to maintain control; consider step down if well controlled for at least three months | Not well controlled: Step up one step and reevaluate in two to six weeks; for adverse effects, consider alternative treatment options | Very poorly controlled: Consider short course of oral systemic corticosteroids; step up one to two steps, and reevaluate in two weeks; for adverse effects, consider alternative treatment options |
ONGOING MANAGEMENT OF ASTHMA
Ongoing management centers on controller medications. These include inhaled corticosteroids and leukotriene receptor antagonists. Theophylline and cromolyn (Intal) are still listed, but these are not preferred agents, and they do not work as well as inhaled corticosteroids or leukotriene receptor antagonists. Inhaled corticosteroids are the fundamental and first-line therapy in ongoing management because of their proven effectiveness and, in recommended doses, few systemic adverse effects. Well-designed studies demonstrate that inhaled corticosteroids improve asthma control more effectively in children and adults than any other single long-term controller medication.15,16
Written action plans detailing medications and environmental control strategies tailored for each patient are recommended for all patients with asthma, and especially for patients with persistent asthma.17–23 Examples of action plans are available at the National Heart, Lung, and Blood Institute Web site (http://www.nhlbi.nih.gov/health/public/lung/asthma/asthma_actplan.htm).24
Planned asthma-care visits are one of the key recommendations of the new guidelines. These visits are essential for adequate teaching and asthma control. Strategies for planned visits have been published.25 Patients with intermittent asthma may need to be evaluated only once yearly. Those on controller agents should be seen at least twice yearly, and as often as every four months.
EXACERBATIONS
One of the major differences from previous guidelines involves reinstating the 199126 cut points of FEV1 or PEF (70 percent or more of predicted FEV1 or PEF) as criteria for discharge from the urgent care setting and identifying patients for whom response to therapy is incomplete and who usually require continued treatment or hospitalization (40 to 69 percent of predicted). The limited value of pulmonary function measures in very severe exacerbations is acknowledged. For home management of acute exacerbations (compared with more gradual decline of control), the EPR-3 report no longer recommends doubling the dose of inhaled corticosteroids.
Final Comments
A vital element in effective early treatment is having a written asthma action plan for patients to guide self-management, including instructions on how to recognize signs of deterioration and warning signs for when to contact one's family physician.