
Am Fam Physician. 2009;80(3):273-279
Author disclosure: Nothing to disclose.
More than 72 million Americans have hypertension, and the majority of these persons have essential hypertension. However, a significant subset has a secondary cause. The most common cause of secondary hypertension is renal vascular hypertension, of which renal artery stenosis is the leading pathology. Up to 5 percent of all occurrences of hypertension are caused by renal artery stenosis, equating to as many as 3.5 to 4 million occurrences in the United States. Detecting renal artery stenosis is particularly important for ensuring that this potentially curable form of hypertension is identified and treated properly. Duplex Doppler ultrasonography is a good screening test in many patients, but it has limitations in larger persons and can overlook small accessory arteries. For patients with normal renal function but a high clinical index of suspicion for renovascular disease, contrast-enhanced magnetic resonance angiography and computed tomographic angiography are the most accurate imaging tests. For patients with diminished renal function, gadolinium-enhanced contrast magnetic resonance angiography is the best imaging test. However, caution is warranted because exposure to gadolinium contrast agents is associated with nephrogenic systemic fibrosis in patients with renal failure. The American College of Radiology has developed appropriateness criteria for imaging tests related to the diagnosis of renal artery stenosis. This article is a summary of the recommendations, with the advantages and limitations of each test.
The prevention and management of hypertension are major public health challenges for the United States. Most patients with hypertension have essential hypertension that is managed with lifestyle adjustments and pharmacologic therapy.1 In some patients, a secondary treatable cause of hypertension is present, with renal artery stenosis as the most common etiology. Although there are a number of diseases that can cause renal artery stenosis, atherosclerosis and fibromuscular dysplasia are the most common.
There are a variety of clinical findings associated with renovascular hypertension (Table 1).2 The presence of these findings improves the predictive value of the diagnostic imaging evaluations. If renal artery stenosis can be reversed, the patient's hypertension can theoretically improve. However, it is important to note that the presence of a detectable stenosis that can be treated with angioplasty or surgical intervention does not ensure a cure for all patients. No definitive clinical or radiographic findings currently exist to indicate which patients will benefit from revascularization. Studies comparing medical therapy with surgical therapy are conflicting.3–6 In addition, there is little evidence that revascularization improves patient-oriented outcomes, such as reducing mortality and morbidity from heart disease and renal disease. Prospective studies are underway.6
| Abdominal bruit |
| Deterioration of renal function in response to angiotensin-converting enzyme inhibitors |
| Generalized arteriosclerotic occlusive disease with hypertension |
| Malignant or accelerated hypertension |
| New onset of hypertension after 50 years of age (suggestive of atherosclerotic renal artery stenosis) |
| Refractory hypertension |
| Significant hypertension (diastolic blood pressure > 110 mm Hg) in a young adult (i.e., younger than 35 years) suggestive of fibromuscular dysplasia |
| Sudden development or worsening of hypertension |
The radiologic tests available for diagnosing renal artery stenosis include computed tomographic angiography, duplex Doppler ultrasonography, magnetic resonance angiography, and nuclear medicine angiotensin converting enzyme (ACE) inhibitor renography. These noninvasive tests have the ability to identify the anatomic stenosis and, in some instances, provide functional information. The most appropriate test for each patient depends on the strengths and weaknesses of the particular test, as well as the patient's clinical findings and renal function at the time of the test. The most appropriate test for a patient with renal insufficiency is often different from the one that should be used for the patient with normal renal function.
Illustrative Cases
CASE 1
A 78-year-old man presented for evaluation of previously controlled hypertension. He had a history of coronary artery disease with two myocardial infarctions and type 2 diabetes. His blood pressure recently became more labile and difficult to control, with an average systolic pressure of 159 mm Hg on an ambulatory 24-hour blood pressure monitor. His physical examination was unremarkable; specifically, no abdominal bruit was identified. His serum creatinine level was 1.6 mg per dL (140 μmol per L). Given his history of vascular disease and recent onset of uncontrollable hypertension, renal artery stenosis was suspected. Because of his mildly elevated creatinine, a duplex Doppler ultrasonography was ordered, followed by magnetic resonance angiography.
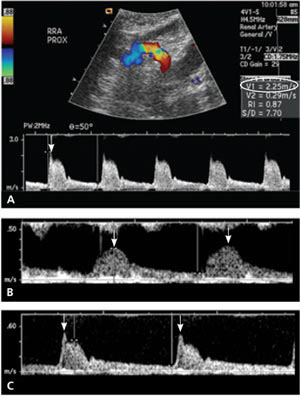
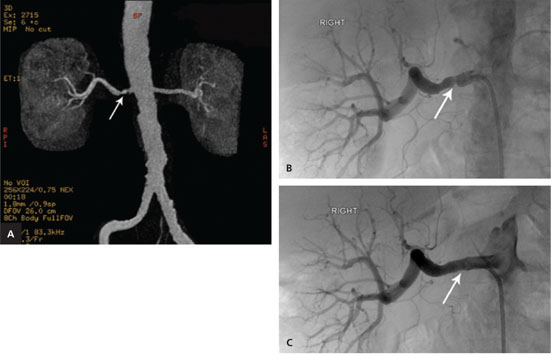
Ultrasonography demonstrated elevated velocities of blood flow of 2.25 m per sec (normal is less than 2.0 m per sec) in the main right renal artery (Figure 1A). The waveforms of the arterial flow in the segmental renal arteries showed a parvustardus waveform (delayed and weak peak-systolic velocity) typical of an upstream stenosis (Figures 1B and 1C). On subsequent magnetic resonance angiography, a focal anatomic stenosis was identified in the main right renal artery (Figure 2A). Catheter-directed renal artery angiography confirmed renal artery stenosis (Figure 2B), and angioplasty was performed, with placement of an arterial stent across the stenosis (Figure 2C). Following reduction of the stenosis, the patient became normotensive, with blood pressure readings in the 120s/60s mm Hg.
CASE 2
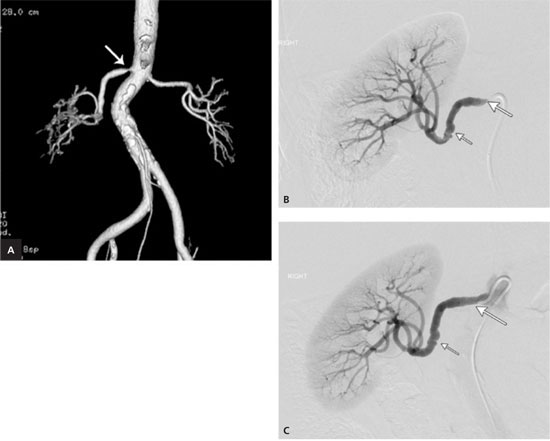
A 69-year-old woman with obesity presented with a five-year history of uncontrolled hypertension, despite multiple medications, and a recent onset of type 2 diabetes. Her blood pressure reading at the time of examination was 212/103 mm Hg. Her physical examination was unremarkable; specifically, no abdominal bruit was identified. Her serum creatinine level was 1.0 mg per dL (90 μmol per L). Given the uncontrollable nature of her hypertension, renal artery stenosis was suspected. Computed tomographic angiography was performed on this patient with normal renal function. This demonstrated a tight ostial (proximal) stenosis that is typical of atherosclerosis (Figure 3A). Catheter-directed renal artery angioplasty with stent placement was performed, and the ostial lesion was identified and treated (Figures 3B and 3C). Fibromuscular dysplasia involving the mid-right renal artery was discovered and treated with balloon angioplasty before the stent placement. Following reduction of the stenoses, the patient's hypertension improved, with subsequent blood pressure readings in the 150s/80s mm Hg.
Tests Indicated for Suspected Renal Artery Stenosis
The following is a discussion of some of the more commonly used noninvasive diagnostic imaging tests for renal artery stenosis, which are summarized in Table 2.2 For a more detailed description of these tests and other less commonly used imaging tests, see the American College of Radiology (ACR) report.2
| Radiologic procedure | Rating* | Comments |
|---|---|---|
| Variant 1: High index of suspicion of renovascular hypertension and normal renal function | ||
| Magnetic resonance angiography of kidney | 8 | Requires intravenous gadolinium contrast agents; accurate in diagnosing renal artery stenosis |
| Computed tomographic angiography of kidney | 8 | Similar to magnetic resonance angiography in accuracy; requires intravenous iodinated contrast media |
| Duplex Doppler ultrasonography of kidney | 6 | Useful if there is a dedicated team of physicians and technologists who are skilled in the test |
| Nuclear medicine ACE-inhibitor renography | 6 | Although the testing technique has not been standardized, it appears to have a relatively high sensitivity and specificity in patients with normal renal function |
| Invasive arteriography of kidney | 4 | Considered the preferred method for diagnosing renal artery stenosis, but it is invasive; probably not indicated as primary diagnostic test, but must be performed before transluminal angioplasty; reserved for confirmation and for angioplasty or stent placement |
| Invasive renal vein renin assays | 3 | Should not be used as a screening test, but rather to confirm the clinical significance of a renal artery stenosis |
| Radiographic intravenous urography | 1 | Significantly less sensitive than other tests |
| Invasive angiography intravenous digital subtraction of kidney | 1 | Difficult to perform on a reliable basis because of a high number of inadequate studies |
| Variant 2: High index of suspicion of renovascular hypertension and diminished renal function | ||
| Magnetic resonance angiography of kidney | 8 | Useful in older patients with arteriosclerotic vascular disease with diminished renal function who most likely have proximal renal artery stenosis |
| Duplex Doppler ultrasonography of kidney | 8 | Reliable if there is a dedicated team of physicians and technologists who are skilled in the test |
| Nuclear medicine ACE-inhibitor renography | 4 | Although diminished renal function can affect the sensitivity and specificity of the test, it is still reliable as a screening test |
| Invasive angiography intravenous digital subtraction of kidney | 4 | Difficult to perform on a reliable basis; requires contrast media |
| Invasive arteriography of kidney | 4 | Better than conventional angiography because it requires less contrast media; it is often used to guide angioplasty or stent placement |
| Invasive renal vein renin assays | 3 | Should not be used as a screening test |
| Radiographic intravenous urography | 2 | Significantly less sensitive than other tests; uses contrast media |
| Invasive angiography of kidney | 1 | Not indicated because of large contrast load to kidneys |
| Computed tomographic angiography of kidney | 1 | Not indicated because of contrast load to kidneys |
| Variant 3: Low index of suspicion of renovascular hypertension (essential hypertension) | ||
| These patients do not require diagnostic imaging tests | ||
COMPUTED TOMOGRAPHIC ANGIOGRAPHY
Computed tomographic angiography involves the process of rapidly acquiring volumetric images by continuously moving the beam in a helical manner across a region of interest during a single bolus infusion of intravenous contrast. However, the use of iodinated contrast raises the risk of nephrotoxicity in patients with pre-existing renal failure. Sophisticated methods of image processing allow three-dimensional (3D) displays of the aorta and renal vasculature that are remarkably clear.
Two studies7,8 comparing computed tomographic angiography with digital renal arteriography have reported the sensitivity of computed tomographic angiography for detecting significant stenoses (greater than 50 percent narrowing) to be 88 to 96 percent and the specificity to be 77 to 98 percent. The accuracy was 89 percent in one study.8 In diagnosing narrowing of only the main renal arteries, one study found the sensitivity and specificity to be 100 and 98 percent, respectively.7 Normal results from computed tomographic angiography virtually rule out renal artery stenosis. Secondary signs include post-stenotic dilatation, renal parenchymal changes of atrophy, and decreased cortical enhancement.9
DUPLEX DOPPLER ULTRASONOGRAPHY
Duplex Doppler ultrasonography is an attractive option because it is a noninvasive test that is relatively inexpensive, does not require contrast medium, and can be used in patients with any level of renal function. As with many of the noninvasive imaging tests, there are numerous parameters and abnormal criteria indicating possible renovascular disease. The most frequently quoted parameters are a peak systolic velocity in the renal artery exceeding 1.8 or 2.0 m per sec and a renal artery/aortic velocity ratio exceeding 3.5.10 Using these parameters, early investigators have quoted sensitivities from 85 to 90 percent. Specificities were also notably high at 90 percent.11 Some reports have advocated additional analysis of the segmental renal artery waveform using measurements such as acceleration time and acceleration index, as well as a parvustardus waveform appearance.12
A major problem with ultrasonography for renal artery stenosis is that approximately 10 to 20 percent of patients may have technically inadequate studies secondary to obesity or overlying bowel gas.13 In addition, examination times can vary from 10 to 15 minutes to up to 1.5 hours based on the skill of the technician and difficulty of the examination secondary to body habitus and the presence or absence of bowel gas. Duplex Doppler ultrasonography is less useful than magnetic resonance angiography for diagnosing fibromuscular dysplasia and detecting accessory renal arteries.14
Because of the difficulty and time involved with duplex Doppler ultrasonography, it should be used in medical centers where it has proven to be reliable and where dedicated technologists and physicians are skilled in this test.
MAGNETIC RESONANCE ANGIOGRAPHY
Magnetic resonance angiography is a noninvasive test for assessing renal artery stenosis and has been widely applied for clinical practice. The reliability of magnetic resonance angiography is not affected by the presence of bilateral renovascular disease. Currently, 3D contrast-enhanced magnetic resonance angiography with an intravenous injection of gadolinium-based contrast agent forms the backbone of magnetic resonance imaging (MRI) of renal arteries.
Several investigators report sensitivities ranging from 88 to 100 percent, and specificities ranging from 71 to 100 percent, using angiography as the standard of reference.15 In a meta-analysis of 39 studies, 25 of which met the inclusion criteria, the sensitivity and specificity of gadolinium-enhanced magnetic resonance angiography were 97 and 85 percent, respectively.16 With the use of high spatial-resolution, small field-of-view magnetic resonance angiography techniques, it is now possible to evaluate not only the main renal arteries, but also the accessory renal arteries and distal stenosis.
Most MRI tests rely solely on the morphologic assessment of the vasculature. To assess the hemodynamic consequences of a particular arterial lesion, additional functional tests are sometimes required.15,17 Initially, gadolinium-based MRI contrast agents were believed to be well tolerated and nonnephrotoxic, even when used in patients with impaired renal function. However, exposure to gadolinium contrast agents in patients with renal failure and those maintained on dialysis has recently been linked with the development of nephrogenic systemic fibrosis, a debilitating and sometimes fatal disease affecting the skin, muscle, and internal organs.18–20 The ACR guidelines for safe MRI practices state that for all patients with moderate to end-stage kidney disease (i.e., estimated glomerular filtration rate [GFR] of less than 60 mL per min per 1.73 m2) and those with acute renal injury, it is recommended that gadolinium contrast agents not be administered unless a risk-benefit assessment for that particular patient indicates that the benefit clearly outweighs the potential risks.19
NUCLEAR MEDICINE ACE-INHIBITOR RENOGRAPHY
Renal scanning with radionuclide agents is noninvasive and safe, even in patients with renal insufficiency. In addition, some reports suggest its ability to identify patients who will benefit from surgical or angioplasty intervention. When using technetium (Tc) 99m mertiatide (MAG3), a renogram curve showing a prolonged time to peak activity and delayed washout suggests renal artery stenosis.21–23
Because an ACE inhibitor significantly reduces the GFR in kidneys with a partial vascular obstruction, using ACE inhibitor-enhanced GFR renography (using diethylenetriamine pentaacetic acid) can be dramatic. Apparently, renal tubular secretion is also greatly affected by the addition of an ACE inhibitor, and iodine 131 (I-131) hippuran and Tc 99m MAG3 are therefore also sensitive. Tc 99m MAG3 provides superior images and counting accuracy compared with I-131 hippuran.
A review of the literature regarding all methods of captopril renography revealed sensitivities generally in the range of 80 to 100 percent.20,22,24 A previous study suggested that captopril renography is helpful in identifying patients who will benefit from surgical or angioplasty intervention based on blood pressure reduction.25 This seems to be more evident with the tubular secretion agents (I-131 hippuran and Tc 99m MAG3). The ability to identify patients who will benefit from surgery or angioplasty is considered highly valuable. The relatively high sensitivity and specificity of this diagnostic imaging test have enabled it to be a primary screening test for renal artery stenosis, especially in patients with normal or near-normal renal function. However, ACE-inhibitor renography is not a reliable test in patients with poor renal function or bilateral renal artery stenoses, even though it is safe for them.
FINAL COMMENTS
Diagnostic imaging tests for suspected renovascular hypertension depend on the index of suspicion for renovascular disease and on the patient's renal function. If clinical findings strongly suggest the possibility of renovascular disease, contrast-enhanced magnetic resonance angiography or computed tomographic angiography should be performed. Duplex Doppler ultrasonography or captopril renography could also be used if magnetic resonance angiography is not desired or is contraindicated. Computed tomographic angiography may be helpful in a select group of patients who are likely to have proximal renal artery stenosis. Conventional angiography and intra-arterial digital subtraction angiography should be reserved for confirmation and therapeutic reasons, such as angioplasty and stent placement, especially with the recent advances in the magnetic resonance angiography and computed tomography tests and their successful results.26,27
Three variants in this guideline are based on the index of suspicion of renovascular disease and on the patient's renal function. The first variant is for those patients with a high index of suspicion of renovascular disease who have normal renal function. In these patients, contrast-enhanced magnetic resonance angiography and computed tomographic angiography are the most accurate means to evaluate for renovascular disease. Captopril renography is also adequate in these patients if magnetic resonance angiography and computed tomographic angiography are not desired or are contraindicated. Duplex Doppler ultrasonography can be used in these patients if a dedicated team of technologists and radiologists is available and the testing technique has proven to be reliable in that medical center.
The second variant includes patients with a high index of suspicion of renovascular disease and diminished renal function. In these patients, gadolinium-enhanced contrast magnetic resonance angiography is best suited to evaluate renovascular disease. However, the recently reported association of exposure to gadolinium contrast agents in patients with renal failure with nephrogenic systemic fibrosis warrants caution. Duplex Doppler ultrasonography is also the preferred screening test, especially in a medical center where the testing technique has proven to be reliable and where dedicated technologists and physicians are skilled in the test and can perform it with a high degree of accuracy. Captopril renography is not a reliable test in patients with poor renal function. Computed tomographic angiography may also be contraindicated secondary to renal insufficiency.
Finally, a third variant includes patients with hypertension and a low index of suspicion of renovascular disease. These patients most likely have essential hypertension that is usually easily controlled with medication. There is no need for diagnostic imaging tests in these patients. When renal artery stenosis is suspected and diagnosed, it is debatable about which patients will benefit from revascularization.