
Am Fam Physician. 2009;80(6):597-602
Author disclosure: Dr. Ho has received in-kind research support for MRI development from GE Healthcare.
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the U.S. Department of Defense or the Uniformed Services University of the Health Sciences.
The population of adults with congenital heart disease is increasing in North America. Radiologic imaging is critical for the initial assessment and for surveillance in this population. Chest radiography and echocardiography are valuable first-line tools for evaluation. However, magnetic resonance imaging and computed tomography are often necessary, particularly for assessment of extracardiac anatomy or specific vascular connections or relationships, which may be complex in postoperative patients. Although magnetic resonance imaging and computed tomography can provide volumetric data for more comprehensive evaluation of cardiac anatomy and function, magnetic resonance imaging does not require patient exposure to ionizing radiation or nephrotoxic iodinated contrast media. Magnetic resonance imaging also can measure blood flow for quantification of left-to-right shunts, regurgitant fractions, and pressure gradients. Although noninvasive imaging techniques have limitations, they can evaluate most lesions and preclude the need for cardiac catheterization. Noninvasive imaging is particularly useful for serial evaluation of patients with surgically corrected congenital heart disease, because nearly one half of these patients will require two or more surgeries.
The number of adults in North America who have congenital heart disease (CHD) has steadily increased over the past 30 years.1,2 Although this increase is partly because of the growth of the adult population, it may be more attributable to improvements in surgical management, which have resulted in a greater than 90 percent 10-year survival rate in persons with CHD.3 Between 1979 and 1997, mortality from CHD decreased 39 percent in the United States.4 In North America, at least 500,000 adults have had surgical correction of CHD, with nearly 50 percent requiring two or more surgeries, and 23 percent requiring three or more.2,3,5
Congenital heart lesions may become symptomatic any time from birth to adulthood. At least 10 percent of persons with CHD have lesions that are typically not diagnosed until adulthood, such as secundum atrial septal defect (ASD) and congenitally corrected transposition of the great arteries (a condition in which the aorta and pulmonary artery are transposed, as are the ventricles, resulting in congenital correction of circulation).1,2 Patients with some types of common CHD lesions often survive into adulthood (e.g., those with bicuspid aortic valve, congenital forms of mitral valve prolapse, coarctation of the aorta, ASDs, pulmonary valve stenosis, patent ductus arteriosus, or tetralogy of Fallot).2
Diagnostic imaging procedures for adults with suspected CHD include chest radiography, echocardiography (transthoracic and transesophageal), nuclear scintigraphy, cardiac-gated computed tomography (CT), magnetic resonance imaging (MRI), and cardiac catheterization and angiography. The evaluation of CHD often requires the accurate assessment of not only intra- and extracardiac anatomy, but also hemodynamics and function. Adults with CHD have the additional clinical considerations of acquired comorbidities (e.g., hypertension, atherosclerosis, occlusive coronary artery disease, pulmonary disease, renal disease) that may complicate their medical or surgical treatment.2
Illustrative Case
A 40-year-old woman presented with a one-year history of worsening dyspnea on exertion that was sometimes associated with vague chest pain. She had no history of cardiopulmonary disease and was not taking any medications. Electrocardiography was nonspecific, and the physical examination was significant only for a mild systolic murmur at her left lower sternal margin. Chest radiography suggested right heart enlargement. Transthoracic echocardiography (TTE) revealed mild to moderate enlargement of the right atrium and right ventricle, and moderate tricuspid insufficiency. Left ventricular function was normal (ejection fraction of 55 to 60 percent). Transesophageal echocardiography (TEE) was performed to evaluate for left-to-right shunt. TEE revealed two ASDs and probable right upper partial anomalous pulmonary venous return (PAPVR). The left superior and inferior pulmonary veins were noted to drain normally to the left atrium, but the right inferior pulmonary vein was not identified.
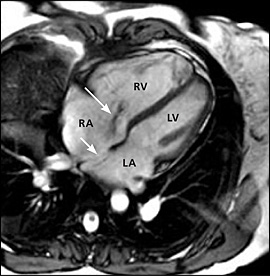
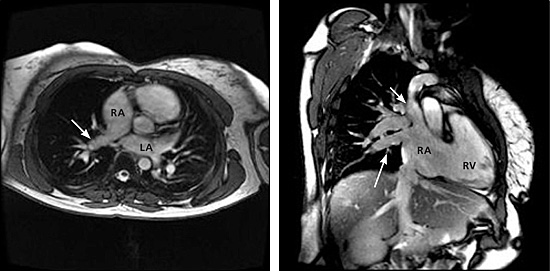
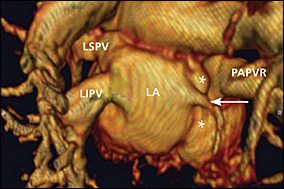
Cardiac MRI was ordered to assess the pulmonary venous drainage (especially the right pulmonary veins) and intracardiac anatomy, to evaluate for additional extracardiac lesions, and to quantify the left-to-right shunt (i.e., pulmonic to systemic blood flow [Qp:Qs] ratio). MRI confirmed the echocardiographic diagnosis of ASDs, right heart enlargement, and tricuspid insufficiency (Figure 1). MRI and gadolinium-enhanced three-dimensional magnetic resonance angiography (MRA) revealed normal left superior and inferior pulmonary veins, and a diminutive right inferior pulmonary vein draining normally to the left atrium, with the remainder of right pulmonary venous return draining via a large right upper PAPVR to the junction of the superior vena cava and right atrium (Figures 2 and 3). MRI showed the Qp:Qs ratio to be greater than 3:1 (i.e., a significant left-to-right shunt). An exercise stress-rest, dual-isotope (thallium 201 and technetium 99m sestamibi) single photon emission CT (SPECT) examination was performed before surgery to exclude the possibility of concomitant occlusive coronary artery disease. The stress-rest SPECT examination was normal, and the patient subsequently underwent surgical closure of the ASDs and rerouting of the right upper PAPVR to the left atrium.
Imaging
The American College of Radiology Appropriateness Criteria for the imaging tests used in the evaluation of adults with suspected CHD are listed in Table 1.6,7 A more detailed discussion has been published previously.7 The illustrative case above highlights an application of imaging used in the evaluation of adults with CHD. Chest radiography was initially performed but was nonspecific, requiring TTE and then TEE for the identification of the underlying ASDs and PAPVR. Cardiac MRI with MRA played an important adjunctive role by delineating the pulmonary venous return, excluding the presence of additional cardiovascular lesions, and quantifying the left-to-right shunt.
| Procedure | Appropriateness rating* | Comments |
|---|---|---|
| Chest radiography | 9 | Recommended in combination with TTE |
| Ultrasound TTE with Doppler | 9 | Recommended in combination with chest radiography |
| Heart MRI | 8 | May be performed as adjunct to TTE by trained operator if additional information is required; see comments regarding contrast in referenced article under “Anticipated Expectations”7 |
| Coronary artery CT angiography | 7 | Preferred technique for suspected coronary anomalies; can also be used for evaluation of coronary artery disease |
| Ultrasound TEE | 7 | May be performed as adjunct to TTE by trained operator if additional information is required |
| Chest magnetic resonance angiography | 6 | To evaluate associated vascular abnormalities; see comments regarding contrast in referenced article under “Anticipated Expectations”7 |
| Heart CT with contrast | 6 | May be an alternative to MRI and TTE or TEE |
| Cardiac catheterization with angiography | 5 | Adjunctive to noninvasive testing, for hemodynamic measurements or coronary artery status, or if other diagnostic information is required |
| Heart nuclear scintigraphy | 4 | May be used for perfusion in patients with suspected ischemic heart disease |
| Nuclear shunt detection | 4 | Alternative to MRI for shunt quantification |
CHEST RADIOGRAPHY
Posteroanterior and lateral chest radiography is widely available and remains a basic first-line imaging technique for adults with suspected CHD. Chest radiography may provide the first suggestion of a congenital lesion, such as a great vessel anomaly.1,8 Chest radiographs can readily illustrate cardiopulmonary and mediastinal contours, pulmonary vascularity, pathologic calcification, and evidence of previous intervention (e.g., metallic surgical clip, valve, stent), but they typically provide insufficient information for definitive diagnosis of CHD.
ECHOCARDIOGRAPHY
TTE is a crucial initial imaging study in adults with suspected CHD. Two-dimensional TTE using color Doppler is well suited for rapid evaluation of cardiac morphology, function, and blood flow. Three-dimensional TTE can supply more information than traditional two-dimensional TTE,9 but it may be non-diagnostic in up to 27 percent of patients.10 TTE has several limitations, such as high operator dependence that diminishes its reproducibility.11 In adults, especially postoperative patients, the acoustic windows may be poor and can preclude adequate CHD assessment.2,12,13 In these situations, TEE and MRI have important roles.
Compared with TTE, TEE can provide a new or alternative diagnosis (14 percent) or new information (56 percent) in adults with CHD.14 Limitations include limited planes of view, poor visualization of specific regions (e.g., apicoanterior septum and right ventricular outflow tract, pulmonary valve, distal right pulmonary artery, proximal left pulmonary artery), and blind areas such as those created by masking of blood flow by implanted prosthetic material.14 TEE is also an invasive examination and is operator-dependent.15
RADIONUCLIDE IMAGING
Historically, radionuclide imaging has been used for the evaluation of ventricular function and quantification of cardiac shunts in adults with CHD.16,17 However, many of these indications are now more readily evaluated with MRI, which provides superior anatomic illustration of CHD lesions and does not require the radiation exposure inherent with radionuclide imaging. In patients who are unable to undergo MRI, nuclear scintigraphy is a viable alternative. As the illustrative case shows, stress-rest radionuclide SPECT imaging is a valuable adjunctive study to exclude concomitant ischemic heart disease.
COMPUTED TOMOGRAPHY
Cardiac-gated CT scanners can quickly evaluate the entire heart and great vessel region as a three-dimensional volume.18–20 Most types of congenital cardiac malformations have been described using this technique. Cardiac-gated CT can provide a variety of cardiac functional measurements, such as ventricular volumes, ejection fraction, regurgitant volumes, and myocardial mass. CT angiography (CTA) can identify congenital abnormalities of the coronary arteries. However, CTA and cardiac CT require exposure to iodinated contrast material and ionizing radiation.
MAGNETIC RESONANCE IMAGING
MRI is particularly useful for evaluating CHD.4,12,13,21–30 MRI can provide essential morphologic and functional information for the management of CHD, without the concerns of ionizing radiation and iodinated contrast material associated with cardiac CT. MRI can show turbulent blood flow (e.g., a flow jet) associated with septal or valvular lesions (Figure 1). Unlike CT, MRI allows direct measurement of blood flow for quantification of left-to-right shunts, regurgitant fractions, and pressure gradients across stenoses and valves.30 Unlike echocardiography, MRI is not limited by an imaging “window,” and images can be obtained in essentially any plane for improved three-dimensional visualization of cardiac anatomy, which can be particularly complex in postoperative patients.25–27
Similar to CT, MRI can provide volumetric coverage of cardiac chambers for determination of cardiac metrics, such as ventricular volumes, ejection fractions, regurgitant volumes, and myocardial mass.
MRI has some contraindications and limitations. Some patients have claustrophobia, but most can tolerate MRI with mild sedation. The presence of certain metallic devices, such as pacemakers, is generally considered a contraindication, although MRI has been performed safely under rigorous safeguards in patients with pacemakers.31 The administration of gadolinium chelate contrast agents may be problematic in some patients, such as those with a severe allergy to these agents or those at significant risk for nephrogenic systemic fibrosis (e.g., renal failure with an estimated glomerular filtration rate of less than 30 mL per minute per 1.73 m2). However, unlike for cardiac CT, the use of contrast agents for MRI evaluation of patients with CHD is often unnecessary. Detection of calcification is a limitation of MRI, so adults with homografts or bioprosthetic valved conduits in which the detection of calcification implies deterioration may not be optimally imaged.
TTE/TEE VS. MRI
MRI often provides information that is additive to that of TTE.13 In some evaluations, MRI can more reliably assess lesion severity and can be more successfully performed than TTE.32 MRI is comparable with TTE in the evaluation of isolated intracardiac defects, but MRI is often more useful in the diagnosis of complex congenital lesions, as well as lesions involving the right ventricular outflow tract or pulmonary arteries, and systemic-to-pulmonary shunts.33–35
TEE is superior to MRI in the evaluation of intracardiac anatomy in adults with CHD, but MRI is superior in assessing extracardiac anatomy.36 The two techniques provide similar overall diagnostic information. However, when TEE and MRI are used in combination, they provide important complementary information in all diagnostic categories.36
CARDIAC CATHETERIZATION AND ANGIOGRAPHY
Cardiac catheterization and angiography have been the diagnostic gold standard for CHD for 50 years. For the past two decades, this technique has been increasingly supplemented by noninvasive diagnostic modalities—initially, echocardiography and, more recently, MRI and cardiac CT. In 2001, a task force of the 32nd Bethesda Conference suggested the use of diagnostic catheterization primarily to resolve specific issues related to surgical intervention, such as the preoperative evaluation of coronary arteries; the assessment of pulmonary vascular disease and its response to vasoactive agents for traditional surgical intervention and/or heart or heart/lung transplantation; and as an adjunct to noninvasive assessment for further delineation of morphologic and functional characteristics of complex congenital lesions.
Although diagnostic cardiac catheterization continues to be performed, many patients with simple congenital cardiac defects now undergo surgery based on non-invasive preoperative evaluation alone. Technical advances in echocardiography, cardiac CT, and MRI have been significant, and further advances will likely expand the roles of these techniques in the evaluation of CHD and diminish the need for invasive diagnostic catheterization and angiography.