
Am Fam Physician. 2009;80(6):609-616
A more recent article on ovarian cancer is available.
Related: Point-of-Care Guide.
Patient information: See related handout on ovarian cancer, written by the authors of this article.
Author disclosure: Nothing to disclose.
Although ovarian cancer may occur at any age, it is more common in patients older than 50 years. Patients often present with nonspecific pelvic or abdominal symptoms. Initial diagnostic tests include transvaginal ultrasonography and serum cancer antigen 125 measurement; however, these tests are not specific for ovarian cancer. Conventional treatment includes surgical debulking followed by chemotherapy. Prognosis is typically determined by the cancer stage and grade, although future treatment may depend on tumor genetic composition. Epithelial ovarian cancer is the most common type of ovarian cancer, and because 70 percent of cases are diagnosed at stage III or IV, it is associated with a poor prognosis. Preventive visits provide an opportunity to identify and educate women at increased risk of ovarian cancer, but routine screening is not recommended. Women with a family history of ovarian cancer or a known associated genetic syndrome should be offered genetic counseling or a discussion of available preventive interventions, respectively.
Ovarian cancer accounts for only 3 percent of cancers in women. However, it is the fifth most common cause of cancer death in women behind lung, breast, colorectal, and pancreatic cancers.1 Ovarian cancer has an age-adjusted incidence of 12.5 per 100,000 women.2 There has been a decline of less than 1 percent in the incidence of ovarian cancer in the previous two decades, and the mortality rate is largely unchanged.3 The incidence of ovarian cancer and mortality rates increase with age. Most cases of ovarian cancer occur in women older than 50 years, but the diagnosis may be made at any age, including infancy (Table 11-3).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The USPSTF recommends against routine screening for ovarian cancer. | A | 11, 14, 38 |
| The USPSTF recommends against routine referral for genetic counseling or routine BRCA testing in women whose family history is not associated with an increased risk of deleterious mutations in BRCA1 or BRCA2 genes. | A | 11, 14, 38 |
| The USPSTF recommends genetic counseling and evaluation for BRCA testing in women whose family history is associated with an increased risk of deleterious mutations in BRCA1 or BRCA2 genes. | A | 11, 14, 38 |
| Prophylactic bilateral salpingo-oophorectomy should be considered in women with a mutation in the BRCA1 or BRCA2 gene, or hereditary nonpolyposis colorectal cancer syndrome (i.e., Lynch II syndrome). | C | 40, 41 |
| For patients with hereditary nonpolyposis colorectal cancer syndrome or hereditary breast and ovarian cancer syndrome who choose not to undergo prophylactic bilateral salpingo-oophorectomy, the National Comprehensive Cancer Network recommends transvaginal ultrasonography and CA 125 measurement every six months during days 1 through 10 of the menstrual cycle. This should begin at 35 years of age, or five to 10 years earlier than the earliest age of diagnosis of ovarian cancer in the family. | C | 43, 44 |
| Age | Incidence | Mortality |
|---|---|---|
| Younger than 20 years | 0.7 | 0.04 |
| 20 to 49 years | 6.6 | 2.0 |
| 50 to 64 years | 26.9 | 16.0 |
| 65 to 74 years | 48.6 | 36.1 |
| 75 years and older | 55.6 | 55.2 |
Risk Factors
The greatest risk factors of ovarian cancer are a family history and associated genetic syndromes (Table 2).4-10 A history of ovarian cancer in one relative raises a woman's lifetime risk to 5 percent; a history in two relatives raises the risk to 7 percent.11 Women with specific gene mutations are at higher risk of ovarian cancer. Hereditary breast and ovarian cancer syndrome, which occurs in one in every 500 women, is an autosomal dominant mutation in the BRCA1 or BRCA2 gene. Hereditary breast and ovarian cancer syndrome increases the risk of breast, ovarian, pancreatic, and prostate cancers, and is associated with a 23 to 54 percent lifetime risk of ovarian cancer (Table 312-14). Overall, hereditary breast and ovarian cancer syndrome accounts for only 12 percent of ovarian cancer occurrences.4 Hereditary nonpolyposis colorectal cancer syndrome (i.e., Lynch II syndrome) is an autosomal dominant mutation that increases the risk of nonpolyposis colorectal, endometrial, breast, and ovarian cancers.5 The lifetime risk of ovarian cancer in carriers of hereditary nonpolyposis colorectal cancer syndrome is 12 percent.4
| Increased risk |
| Delayed childbearing |
| Early menarche |
| Endometriosis |
| Estrogen replacement therapy for more than five years |
| Family history suggesting genetic predisposition |
| Genetic syndromes |
| High-fat diet |
| Late menopause |
| Low parity |
| Decreased risk |
| Breastfeeding for 18 months or more |
| Early menopause |
| Multiparity (risk decreases with each additional pregnancy) |
| Hysterectomy* |
| Late menarche |
| Low-fat diet |
| Oral contraceptive use |
| Tubal ligation* |
| Ashkenazi Jewish women* |
| One first-degree relative with breast or ovarian cancer |
| Two second-degree relatives on the same side of the family with breast or ovarian cancer |
| All other women |
| Two first-degree relatives with breast cancer, one of whom was diagnosed by 50 years of age |
| Three or more first- or second-degree relatives with breast cancer |
| A combination of breast and ovarian cancers among first- and second-degree relatives |
| One first-degree relative with bilateral breast cancer |
| Two or more first- or second-degree relatives with ovarian cancer |
| One first- or second-degree relative with the combination of breast and ovarian cancer |
| Breast cancer in a male relative |
Repeated ovulatory rupture and repair theoretically creates opportunities for malignant gene mutations. This may explain the apparent protective effects of oral contraceptives, late menarche, early menopause, multiparity, and breastfeeding. Each of these factors decreases the occurrence of ovulation.6 Increased progesterone levels may also explain the protective effect of multiparity and oral contraceptive use.7
The Women's Health Initiative Dietary Modification Randomized Controlled Trial demonstrated decreased ovarian cancer risk in postmenopausal women after four years of a low-fat diet.8 Although previous research has suggested a relationship between perineal talc use and increased risk of ovarian cancer,15 a recent meta-analysis found no significant link.16 Several modifiable risk factors (e.g., obesity, smoking, a high-starch or high-fat diet, sedentary lifestyle) are associated with an increased risk of ovarian cancer, but have not been established as causes of ovarian cancer. Increased daily fiber intake; the use of carotene, vitamin C, vitamin E, and unsaturated fatty acids; and increased physical activity are moderately associated with a decreased risk of ovarian cancer.9 However, several confounding factors may coexist, and there is limited evidence to support recommending specific lifestyle modifications to reduce ovarian cancer risk. Most women diagnosed with ovarian cancer have no family history and unknown etiology.
Types and Characteristics
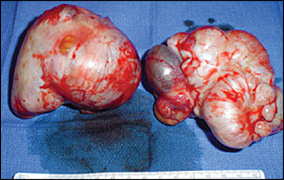
Ovarian cancer tends to develop from three kinds of tissue: approximately 85 to 95 percent from epithelial cells (Figure 1), 5 to 8 percent from stromal cells, and 3 to 5 percent from germ cells.17 The type of ovarian tumor varies depending on the patient's age (Table 417,18). Epithelial cell tumors usually occur in women older than 50 years. Stromal cell tumors may occur in women of any age, although certain tumors, such as androblastomas, may be more common in adolescence. Germ cell tumors usually occur in patients younger than one year and in patients 15 to 19 years of age.2
| Cancer type | Percentage of all ovarian cancers | Characteristics | |
|---|---|---|---|
| Epithelial cell | 85 to 95 | Most common in patients older than 50 years; 15 percent of epithelial cell ovarian cancers are borderline or have low malignant potential, with a 10-year survival rate up to 99 percent for stage I | |
| Serous | 40 percent of all ovarian cancers; most common ovarian cancer | ||
| Endometrioid | 20 percent of all ovarian cancers; 15 percent of endometrioid carcinomas coexist with endometriosis; 40 percent bilateral | ||
| Mucinous | 25 percent of all ovarian cancers; origin unclear; may occur in association with endometriosis; associated with pseudomyxoma peritonei* | ||
| Stromal cell | 5 to 8 | Derived from the sex cord of embryonic gonads | |
| Granulosa-theca | Wide age range; may produce precocious sexual development in prepubertal girls; may be associated with endometrial hyperplasia, cystic disease of the breast, and endometrial carcinoma in adults; ascites in 40 percent of fibromathecoma tumors; may be associated with ascites and hydrothorax (Meigs syndrome) | ||
| Sertoli-Leydig (androblastomas) | Common in adolescence; may be masculinizing; may block normal female sexual development | ||
| Germ cell | 3 to 5 | Found mostly in children and young adults 20 to 30 years of age; highly malignant; usually unilateral | |
| Endodermal sinus tumor | Most common germ cell ovarian cancer in children; usually larger than 15 cm; median age of patients is 18 years, one third of patients are premenarchal | ||
| Embryonal (multipotential) | Extraembryonic: yolk sac carcinoma | ||
| Primitive: embryonal carcinoma (highly malignant), precocious puberty | |||
| Somatic: immature teratoma | |||
| Trophoblast: choriocarcinomas | |||
| Undifferentiated: dysgerminomas (most common malignant germ cell ovarian cancer), 10 to 20 percent bilateral, radiosensitive | |||
| Mature | Mostly benign, dermoid cysts | ||
| Metastasis to ovaries (Krukenberg tumor) | 5 percent | Typically from breast or gastrointestinal primary sites | |
Ovarian cancer primarily spreads locally to the opposite ovary and the uterus, and then intraperitoneally. Distant metastases are rare, but may occur in the liver, lungs, pleura, adrenal glands, and spleen.17 Table 517,19-24 lists the International Federation of Gynecology and Obstetrics cancer stages and survival rates.24 The histologic grade of the tumors can also affect treatment and prognosis. Low-grade tumors with cells of low malignant potential (i.e., borderline tumors) have a more favorable prognosis than high-grade tumors.
| Stage* | Spread | Five-year survival rate |
|---|---|---|
| I | Limited to ovaries | 90 percent |
| II | Pelvic extension | 60 to 80 percent |
| III | Peritoneal implants and/or retroperitoneal or inguinal lymph nodes | 20 percent |
| IV | Distant metastases | Less than 10 percent |
Diagnostic Evaluation
HISTORY
The diagnosis of ovarian cancer may be difficult because of nonspecific symptoms (Table 618,25-30). The most common presenting symptom in children and adolescents is abdominal pain, although precocious puberty, irregular menses, or hirsutism may also be present.18 Patients with ovarian cancer may have abdominal pain, swelling, or nonspecific gastrointestinal symptoms for more than six months before diagnosis. One study evaluating Medicare claims in women older than 68 years found that the most common symptom before diagnosis of ovarian cancer was abdominal pain.25 This study also found that women were more likely to have abdominal imaging than pelvic imaging or cancer antigen (CA) 125 testing.25 These findings emphasize the need to consider ovarian cancer in the differential diagnosis of unresolved abdominal symptoms in postmenopausal women.
| Family history |
| Bilateral breast cancer |
| Early-onset breast cancer (younger than 50 years) |
| Male breast cancer |
| Personal or family history of colon or endometrial cancer |
| Two or more first- or second-degree relatives with ovarian cancer |
| Symptoms |
| Abdominal fullness |
| Back pain |
| Constipation |
| Diarrhea |
| Early satiety |
| Fatigue |
| Nausea |
| Pelvic pain |
| Urinary symptoms |
| Physical findings |
| Abdominal mass |
| Inguinal lymphadenopathy |
| Pelvic or adnexal mass |
| Sister Mary Joseph nodule* |
Another study examined the presenting symptoms in women older than 60 years for up to 12 months before a diagnosis of ovarian cancer.26 Patients with gastrointestinal symptoms (e.g., nausea, vomiting, constipation, diarrhea) had a later-stage cancer diagnosis and a longer lapse between presentation and diagnostic evaluations, compared with patients with gynecologic symptoms (e.g., abnormal vaginal bleeding, genital organ pain).26
Although a single abdominal, genitourinary, or gynecologic symptom is nonspecific for ovarian cancer, a symptom index has been proposed based on retrospective data of initial symptoms in women diagnosed with ovarian cancer.27 The presence of one of six symptoms (i.e., pelvic pain, abdominal pain, increased abdominal size, bloating, difficulty eating, and early satiety), occurring more than 12 days per month for less than one year, has a 56.7 percent sensitivity for early-stage ovarian cancer and a 79.5 percent sensitivity for advanced-stage ovarian cancer.27
PHYSICAL EXAMINATION
Pelvic examination is not sensitive for detecting ovarian masses.28 When a patient presents with any of the historical features listed in Table 618,25-30, a pelvic examination (on an empty bladder) should be performed, as well as a rectovaginal examination to maximize the opportunity to detect a mass. If a pelvic mass is detected, the physician should recognize features that elevate concern, such as a significantly enlarged ovary (greater than 10 cm), ovarian irregularity, a nodular or fixed pelvic mass, bilateral lesions, and nodules in the cul-de-sac.28 Women three to five years after menopause who have a palpable ovary on pelvic examination should also be evaluated, because ovaries normally decrease in size and become nonpalpable after menopause.31 Although patients with ovarian cancer may present with inguinal lymphadenopathy, it is more common for the cancer to spread through retroperitoneal lymph nodes. Patients with advanced disease may have cachexia, ascites, or pleural effusion.30 Table 7 lists the differential diagnosis of adnexal masses.28,29,31-34
| Benign ovarian tumor |
| Cancer metastases |
| Endometrioma |
| Endometriosis |
| Functional ovarian cyst |
| Ovarian torsion |
| Pedunculated uterine fibroid |
| Pelvic kidney |
| Tubo-ovarian abscess |
LABORATORY TESTING
Because the history and examination are often non-specific for ovarian cancer, further testing should be performed in women older than 40 years who have persistent unexplained gynecologic or gastrointestinal symptoms. Laboratory tests in these patients should include a complete blood count, comprehensive metabolic panel, and measurement of serum CA 125 levels. Interpreting CA 125 test results may be difficult because other conditions and physiologic states may be associated with an increased CA 125 level, including pelvic inflammatory disease, endometriosis, functional ovarian cysts, menstruation, and pregnancy.31 Disease processes associated with ascites and other malignancies may also alter CA 125 levels.35
CA 125 is not useful in identifying other cancer types because it is primarily produced in response to an epithelial ovarian cancer cell line. CA 125 levels are elevated in 90 percent of patients with advanced disease, but in only 50 percent of those with stage I tumors.29 In distinguishing between benign and malignant masses, CA 125 has a sensitivity of 61 to 90 percent, a specificity of 71 to 93 percent, a positive predictive value of 35 to 91 percent, and a negative predictive value of 67 to 90 percent.32 Sensitivities, specificities, and positive predictive values at the highest end of these estimates are more applicable to postmenopausal women with a known adnexal mass. The American College of Obstetricians and Gynecologists recommends referral to a gynecologic oncologist for patients with CA 125 levels greater than 200 units per mL (200 arb units per L) in premenopausal women and greater than 35 units per mL (35 arb units per L) in postmenopausal women.32
Increased levels of beta subunit of human chorionic gonadotropin, serum alphafetoprotein, neuron-specific enolase, and lactate dehydrogenase occur in patients with germ cell tumors, and are primarily used to evaluate adnexal masses found during adolescence or young adulthood. Other laboratory biomarkers are available but not commonly used by primary care physicians.
IMAGING
Doppler transvaginal ultrasonography is used in the initial evaluation of adnexal masses. The diagnostic role of other radiologic modalities is limited (Table 8).33,34 Sonographic features suggestive of cancer include complexity with solid and cystic areas, extramural fluid, echogenicity, wall thickening, septa, and papillary projections. Smooth-walled, sonolucent masses are usually benign. Malignant lesions may display an increased number and tortuosity of vessels on Doppler evaluation.33 If ultrasonography reveals suspicious properties, gadolinium-enhanced magnetic resonance imaging may be useful for further evaluation.34
| Modality | Sensitivity* (%) | Specificity* (%) | Use |
|---|---|---|---|
| Transvaginal ultrasonography | 86 | 91 | First-line detection and characterization of adnexal masses |
| Magnetic resonance imaging | 91 | 88 | Further delineation of indeterminate masses seen on ultrasonography |
| Computed tomography | 90 | 75 | Preoperative evaluation |
| Treatment follow-up | |||
| Positron emission tomography | 67 | 79 | Evaluation of increased cancer antigen 125 in known ovarian cancer (e.g., recurrence) with nondetectable implants |
| Detection of metastases in known ovarian cancer, in combination with computed tomography |
Treatment
Conventional therapy for ovarian cancer is surgical tumor cytoreduction followed by a combination of platinum and nonplatinum (taxane-based) chemotherapy, such as carboplatin (formerly Paraplatin) and paclitaxel (Taxol), respectively. There is no reliable evidence that chemotherapy before debulking surgery is superior to conventional treatment in women with advanced epithelial ovarian cancer.36 Surgical staging involves total abdominal hysterectomy, bilateral salpingo-oophorectomy (BSO), and the removal of pelvic and para-aortic lymph nodes and omentum; as well as other supplemental procedures, such as appendectomy, when indicated.19 Appendectomy is a typical supplemental procedure for mucinous ovarian cancer because of the possibility of metastasis.
Ovarian cancer with low malignant potential typically occurs in women 30 to 50 years of age. It presents at stage I in 82 percent of patients and has a survival rate of up to 99 percent.20 This favorable prognosis allows for consideration of conservative fertility-sparing measures, such as unilateral salpingo-oophorectomy. Surgical cytoreduction is considered therapeutic, and adjuvant chemotherapy is reserved for patients with postoperative residual disease or identifiable invasive implants.19 Because of the possibility of late recurrence, surgical treatment completion with total abdominal hysterectomy and unilateral salpingo-oophorectomy should be considered after the reproductive years. Radiation is most often used for palliative purposes or for localized persistent disease after chemotherapy.
Prognosis
Up to 70 percent of patients with epithelial ovarian cancer present at stage III or IV.21 Prognosis is strongly associated with the stage at diagnosis, but the histologic grade also plays a prognostic role, particularly in predicting recurrence.37 Epithelial ovarian cancer is histologically determined to be of low malignant potential in 15 percent of patients, is often diagnosed at stage I, and has a 95 to 99 percent 10-year survival rate.22 Poor prognostic factors are summarized in Table 9.22,23
| Age older than 65 years |
| Clear cell or mucinous tumors (histology) |
| Extensive disease (advanced stage) |
| Large residual tumor volume |
| Lower global quality-of-life score |
| Poor cell differentiation (high grade) |
Prevention and Screening
The CA 125 test is a poor screening test because it does not rule out ovarian cancer, and a false-positive test may lead to unnecessary invasive interventions. The U.S. Preventive Services Task Force (USPSTF) recommends against routine screening for ovarian cancer.11 The USPSTF also recommends against routine referral for genetic counseling or routine BRCA testing in women whose family history is not consistent with increased risk of BRCA1 or BRCA2 mutations.14 The USPSTF recommends referral for genetic counseling and evaluation for BRCA testing in women whose family history is associated with an increased risk of BRCA1 or BRCA2 mutations.14
Annual transvaginal ultrasonography and CA 125 measurement may detect ovarian cancer at an earlier stage in women at high risk of ovarian cancer, but are ineffective at detecting ovarian cancer early enough to improve clinical outcomes.38 A randomized controlled trial of more than 200,000 postmenopausal women in the United Kingdom evaluated the use of CA 125 (interpreted using an ovarian cancer risk algorithm) as an annual screening test, with transvaginal ultrasonography as a second-line test.39 This multimodal screening was compared with annual transvaginal ultrasonography alone. For all primary ovarian and tubal cancers, the sensitivity, specificity, and positive predictive values of multimodal screening were 89.4, 99.8, and 43.3 percent, respectively.39 Longitudinal results of the effect of multimodal screening on mortality may help shape future recommendations. Physicians should consider creating standardized guidelines to help patients at high risk of ovarian cancer make an informed decision about screening. These may include information about the current limited evidence for patients in lower risk categories.
The median age of diagnosis of ovarian cancer associated with BRCA1 mutation is mid-40s, compared with mid-60s for BRCA2.12 This can shape the timing of surgical preventive measures or influence index of suspicion. Prophylactic BSO is effective for reducing the risk of ovarian, fallopian tube, and peritoneal cancers in women with BRCA mutations. If 36 women with BRCA mutations undergo a BSO, one case of associated ovarian cancer will be prevented in 3.5 years.40 For women with hereditary nonpolyposis colorectal cancer syndrome, 28 BSOs would have to be performed to prevent one case of ovarian cancer.41 Total abdominal hysterectomy with BSO should be considered in patients with hereditary nonpolyposis colorectal cancer syndrome because of a 60 percent lifetime risk of endometrial cancer.42
For women at high risk of ovarian cancer who choose not to undergo prophylactic BSO, the National Comprehensive Cancer Network (NCCN) currently recommends transvaginal ultrasonography and CA 125 measurement every six months during days 1 through 10 of the menstrual cycle.43 This should begin at 35 years of age, or five to 10 years earlier than the earliest age of diagnosis of ovarian cancer in the family.43 For hereditary nonpolyposis colorectal cancer syndrome carriers, the NCCN recommends annual transvaginal ultrasonography.44 In one study examining BRCA mutation carriers, semi-annual transvaginal ultrasonography and CA 125 measurement were associated with 71 percent sensitivity and 91 percent specificity for ovarian cancer.45 Other studies of similar patient populations have failed to reproduce these results, often showing sensitivities of less than 50 percent.46