
Am Fam Physician. 2009;80(7):711-714
Author disclosure: Nothing to disclose.
Nephrogenic systemic fibrosis is a progressive, potentially fatal multiorgan system fibrosing disease related to exposure of patients with renal failure to the gadolinium-based contrast agents used in magnetic resonance imaging. Because of this relationship between nephrogenic systemic fibrosis and gadolinium-based contrast agents, the U.S. Food and Drug Administration currently warns against using gadolinium-based contrast agents in patients with a glomerular filtration rate less than 30 mL per minute per 1.73 m2, or any acute renal insufficiency related to the hepatorenal syndrome or perioperative liver transplantation. There have been reports of nephrogenic systemic fibrosis developing in patients not exposed to gadolinium-based contrast agents, but most patients have the triad of gadolinium exposure through contrast-enhanced magnetic resonance imaging, renal failure, and a proinflammatory state, such as recent surgery, endovascular injury, or sepsis. Development of nephrogenic systemic fibrosis among patients with severe renal insufficiency following exposure to gadolinium-based contrast agents is approximately 4 percent, and mortality can approach 31 percent. The mechanism for nephrogenic systemic fibrosis is unclear, and current treatments are disappointing. Prevention with hemodialysis immediately following gadolinium-based contrast agents has been recommended, but no studies have shown this to be effective. Because of the large number of patients with clinically silent renal impairment and the serious consequences of nephrogenic systemic fibrosis related to gadolinium exposure, physicians should use alternative imaging modalities for patients who are at risk.
Nephrogenic systemic fibrosis was first noted in 1997 and reported to the medical community in 2000 as a scleromyxedema-like cutaneous disease seen in patients with renal failure, predominately those on hemodialysis.1 Initially, nephrogenic systemic fibrosis was named nephrogenic fibrosing dermopathy because it was believed the fibroses were limited to the skin. Subsequent postmortem evaluations revealed the fibroses extending into other organ systems.2 The disease was then renamed nephrogenic systemic fibrosis. Although it was initially noted to be a disease in patients with severe renal dysfunction (often those on hemodialysis), it was also noted that patients often had other proinflammatory events, including recent major surgery, thrombosis, malignancy, or infection.3 In early 2006, the relationship between nephrogenic systemic fibrosis and gadolinium-based contrast agents used in magnetic resonance imaging (MRI) was first reported.4 This relationship has been confirmed in subsequent studies, and gadolinium has even been discovered in skin biopsies of patients with nephrogenic systemic fibrosis.5,6
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The U.S. Food and Drug Administration currently recommends against using gadolinium-based contrast agents in patients with a GFR less than 30 mL per minute per 1.73 m2, including patients on peritoneal dialysis or hemodialysis, or with any acute renal insufficiency related to the hepatorenal syndrome or in perioperative liver transplantation, due to the potential for nephrogenic systemic fibrosis. | C | 7 |
| Gadolinium-based contrast agents should never be used as a method of avoiding nephropathy associated with iodinated contrast media. | C | 15 |
| There is no evidence that immediate hemodialysis or initiating hemodialysis for the sole purpose of removing gadolinium-based contrast agents protects against nephrogenic systemic fibrosis. | C | 15 |
| Risks of inducing nephrogenic systemic fibrosis must be weighed against denying MRI enhanced with gadolinium-based contrast agents to patients in need. | C | 7, 15 |
| All patients undergoing MRI enhanced with gadolinium-based contrast agents should be screened for chronic kidney disease by history or laboratory evaluation. | C | 7 |
| Gadolinium-based contrast agents should be used with caution in any patients with a GFR less than 60 mL per minute per 1.73 m2. | C | 15 |
Because of the connection between gadolinium and nephrogenic systemic fibrosis, the U.S. Food and Drug Administration (FDA) currently recommends against using gadolinium-based contrast agents in patients with acute or chronic renal insufficiency, with a glomerular filtration rate (GFR) less than 30 mL per minute per 1.73 m2, or with any acute renal failure caused by the hepatorenal syndrome or perioperative liver transplantation, unless the diagnostic information is essential and not available with noncontrast-enhanced MRI.7 The incidence of nephrogenic systemic fibrosis in patients with severe renal insufficiency following exposure to gadolinium-based contrast agents appears to be approximately 4 percent, without any regard to sex, race, or age.3,8 Proposed risk factors include severe renal dysfunction, acute renal failure, proinflammatory states (e.g., major surgery, thrombotic events, infection, malignancy), and high-dose erythropoietin use.9 Case reports of nephrogenic systemic fibrosis in patients without any exposure to gadolinium have recently been reported.10
Clinical Presentation and Diagnosis
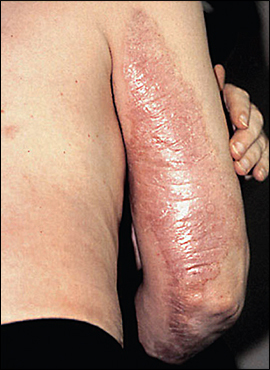
Patients develop symptoms of nephrogenic systemic fibrosis from the day of exposure to gadolinium-based contrast agents to several months following. In one study, the median time to symptoms was 11.5 days after exposure.11 Patients present with pruritic, erythematous plaques with associated induration and edema (Figure 12). The plaques may coalesce to form a peau d'orange appearance. The skin manifestations are symmetric, typically involve the extremities and trunk, and rarely affect the face. Joint contractures are common and often lead to significant disability. The fibrosis is not limited to the skin and can affect multiple organs, leading to multisystem organ failure. Nephrogenic systemic fibrosis also causes respiratory failure from diaphragmatic involvement that may lead to death. Mortality has been reported to be 31 percent, but the true mortality is likely not known because of underreporting and possible misdiagnosis.11
The diagnosis of nephrogenic systemic fibrosis is made by high clinical suspicion in at-risk patients and confirmed by the characteristic findings on skin biopsy. It is recommended that the biopsy extend into the subcutaneous fat or fascia by incisional or deep punch biopsies.3 Biopsy often demonstrates increased CD34 spindle-shaped fibrocytes with thickened collagen bundles. Nephrogenic systemic fibrosis is a rare condition, and cases should be reported to the International Center for Nephrogenic Fibrosing Dermopathy Research at Yale University (http://www.icnfdr.org; e-mail: registermc@juno.com), which contains a registry of more than 300 biopsy-proven cases.12
Risk Factors and Pathophysiology
Many patients with nephrogenic systemic fibrosis have the triad of renal impairment, a proinflammatory state, and gadolinium exposure (Table 1). Most patients have stage 4 (GFR less than 30 mL per minute per 1.73 m2) or worse renal impairment, or are on hemodialysis or peritoneal dialysis, but two case reports of nephrogenic systemic fibrosis in patients with a GFR greater than 30 mL per minute per 1.73 m2 have been reported.11 Both cases involved patients with acute renal failure, making the estimate of GFR less accurate because it was based on a single serum creatinine level. Patients undergoing peritoneal dialysis appear to be at the highest risk, with an incidence of 4.6 cases per 100 patients (versus 0.61 cases per 100 patients undergoing hemodialysis).13 The increased risk may be due to poor clearance of gadolinium by peritoneal dialysis or to a higher rate of MRI being ordered for these patients in an attempt to avoid the nephrotoxicity of iodinated intravenous computed tomography (CT) contrast media.14 The European Society of Urogenital Radiology (ESUR) published guidelines in 2007 that recommend against using gadolinium-based contrast agents in at-risk patients to avoid the nephropathy associated with iodinated contrast media.15
| Gadolinium exposure | |
| Proinflammatory state | |
| Infection (pneumonia, osteomyelitis, sepsis) | |
| Ischemic event | |
| Limb injury | |
| Major tissue injury or trauma | |
| Malignancy | |
| Recent surgery | |
| Thrombosis | |
| Renal impairment | |
| Acute kidney failure | |
| Chronic kidney failure (stage 4 or 5 [glomerular filtration rate < 30 mL per minute per 1.73 m2]) | |
| Hemodialysis | |
| Peritoneal dialysis | |
The pathophysiology behind nephrogenic systemic fibrosis is unclear. Gadolinium, like most heavy metals, is toxic and, therefore, is chelated when administered as a contrast medium for MRI. The stability of the gadolinium and chelate combination varies with different gadolinium-based contrast agents. One proposed theory for nephrogenic systemic fibrosis is the transmetallation of the gadolinium and chelating agent. Transmetallation refers to another element displacing gadolinium from the chelate and forming a free gadolinium ion. This free gadolinium ion may then deposit in tissues. Proinflammatory states likely make the gadolinium more likely to undergo transmetallation and become the damage-causing ion. Renal failure increases the duration of the gadolinium exposure through decreased clearance of free gadolinium. This in turn leads to a higher incidence of deposition into tissue, which explains why nephrogenic systemic fibrosis risk may increase with an increased cumulative dose. This deposition leads to the recruitment of fibrocytes, which, along with the proinflammatory state, causes tissue injury and further fibrocyte recruitment, ultimately leading to nephrogenic systemic fibrosis.
Treatment and Prevention
Currently, there is no effective treatment for nephrogenic systemic fibrosis. Trials with corticosteroids, photopheresis, plasmapheresis, thalidomide (Thalomid), thiosulfate, and methotrexate have not had consistent success.16–20 Hemodialysis can be effective at removing gadolinium contrast media from the body, but there is no evidence that immediate hemodialysis protects against the development of nephrogenic systemic fibrosis.15 Patients already being dialyzed should have a dialysis session scheduled after the gadolinium contrast examination. However, initiating hemodialysis solely to prevent nephrogenic systemic fibrosis is not recommended.15
Prevention of nephrogenic systemic fibrosis is the best practice. It was estimated that approximately 40 to 50 percent of patients who have MRIs receive gadolinium as a contrast medium, although recognition of nephrogenic systemic fibrosis has likely reduced the use of gadolinium-based contrast agents.15 For example, primary care physicians commonly order gadolinium contrast-enhanced MRIs to investigate possible intracranial masses and to study the vasculature of the brain, neck, and abdomen. Thus, the risk of nephrogenic systemic fibrosis can be reduced by judicious use of these scans. As previously mentioned, the FDA recommends that gadolinium-based contrast agents be avoided unless the diagnostic information is essential and not available with noncontrast-enhanced MRI.7 The FDA also recommends against using gadolinium-based contrast for patients with a GFR less than 30 mL per minute per 1.73 m2 or acute renal failure of any severity associated with hepatorenal syndrome or in the perioperative liver transplantation period. The FDA does not make distinctions of which type of gadolinium to avoid, but recognizes that the highest reported agent has been gadodiamide (Omniscan).7 The ESUR guideline on nephrogenic systemic fibrosis specifically discusses the different types of gadolinium contrast media, recommending against using gadodiamide, gadopentetate dimeglumine (Magnevist), and gadoversetamide (Optimark) in patients with stage 4 or 5 chronic kidney disease and those patients with reduced renal function who are awaiting liver transplantation.15 Screening all patients for renal dysfunction by obtaining a history or ordering laboratory testing for GFR calculation is also recommended.7 The ESUR guideline further recommends caution in patients with stage 3 chronic kidney disease (GFR less than 60 mL per minute per 1.73 m2), pregnant women, and children younger than one year.15
Final Comment
Nephrogenic systemic fibrosis is an important disease of which all physicians who order MRI with gadolinium-based contrast agents must be aware. Although the nephrotoxicity of intravenous contrast media for CT scans is well known, the potentially fatal effects of gadolinium-based contrast agents also need to be recognized throughout the medical community. This disorder is still in its infancy of understanding, and further research is needed. Given the large number of MRI scans with gadolinium-based contrast agents performed and the estimated 20 million Americans with chronic kidney disease, all family physicians need to be aware of nephrogenic systemic fibrosis and try to find an alternative imaging modality for those at risk.21