
Am Fam Physician. 2009;80(7):717-723
Patient information: See related handout on bladder cancer, written by the authors of this article.
Author disclosure: Nothing to disclose.
Bladder cancer is the sixth most prevalent malignancy in the United States. The most common type of bladder cancer is urothelial (transitional cell) carcinoma, and cystoscopy remains the mainstay of diagnosis and surveillance. Fluorescence cystoscopy offers improvement in the detection of flat neoplastic lesions, such as carcinoma in situ. Non-muscle-invasive bladder cancer is typically managed with transurethral resection and perioperative intravesical chemotherapy. Intravesical bacille Calmette-Guérin therapy is preferred over mitomycin for those at high risk of disease progression. For muscle-invasive disease, standard management is radical cystectomy. In these patients, neoadjuvant chemotherapy or postoperative adjuvant chemotherapy should be considered based on pathologic risks, such as positive lymph nodes or pathologic T stage. Multidrug systemic chemotherapy involving cisplatin is commonly used. No major organization recommends screening for bladder cancer.
Bladder cancer is the second most common genitourinary malignancy.1 It is the sixth most prevalent malignancy in the United States, accounting for approximately 7 percent of cancers in men and 3 percent of cancers in women.1,2 Bladder cancer ranges from mild disease with a low mortality rate to extremely high-grade tumors associated with high mortality. It has a clear correlation with environmental exposures, such as smoking. Accurate staging and grading is important to select optimal treatment. This article briefly reviews the epidemiology, pathology, diagnosis, and treatment of bladder cancer, with emphasis on recent developments.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The U.S. Preventive Services Task Force recommends against routine screening for bladder cancer in adults. | A | 24 |
| Patients with symptoms of bladder cancer should be evaluated with cystoscopy and bladder wash cytology. | C | 14 |
| Tumor markers should not be used for diagnosis of bladder cancer. | C | 23 |
| Fluorescence cystoscopy may be used to enhance the detection of flat neoplastic lesions, such as carcinoma in situ. | C | 18 |
| Complete blood count, blood chemistry tests, liver function tests, chest radiography, and CT or magnetic resonance imaging of the abdomen and pelvis should be included in the metastatic workup for invasive bladder cancer. | C | 12 |
| All patients with bladder cancer should have an evaluation of the upper urinary tract with intravenous urography, renal ultrasonography, CT urography, or magnetic resonance urography. | C | 21, 22 |
| Urine cytology should be used to identify high-grade tumors and monitor patients for persistent or recurrent disease. | C | 19, 20 |
Epidemiology
Bladder cancer is the fourth most common cancer in men and the eighth most common in women. In 2007, approximately 50,000 men and 17,000 women were diagnosed with bladder cancer, and there were about 14,000 deaths caused by this disease.1 It is primarily found in older persons, with approximately 80 percent of new cases occurring in persons 60 years or older.2 Bladder cancer is about three times more common in men (one in 27) than women (one in 85). It is more prevalent in white persons; however, because of delayed diagnosis, mortality rates are higher in black persons.3
Risk Factors
The best known behavioral risk factor for bladder cancer is cigarette smoking; it accounts for 50 percent of cases in developed countries.4 Smokers have a four to seven times greater risk of developing bladder cancer than nonsmokers.5,6 In industrialized countries, 5 to 10 percent of bladder cancers are caused by occupational exposures, such as aromatic amines used in the manufacturing of chemical dyes and pharmaceuticals and in gas treatment plants.7,8
Schistosoma haematobium infection, endemic to 50 countries in Africa and the Middle East, is another established risk factor. Seventy percent of S. haematobium-related cancers are squamous cell carcinomas, and the remainder are urothelial (transitional cell) carcinomas.9 Exposure of the bladder to radiation, often as treatment for other pelvic malignancies, increases the risk of bladder cancer five to 10 years after treatment. These cancers tend to be high grade and locally advanced.7 Familial clustering of bladder cancer, especially in relatively young persons, suggests genetic involvement in some cases.8,10 Risk factors for bladder cancer are listed in Table 1.
| Behavioral |
| Cigarette smoking |
| Chemical exposure |
| Arsenic in well water |
| Chronic infection |
| Bladder calculi, chronic bladder infection, genitourinary tuberculosis, long-term indwelling catheter, schistosomiasis |
| Iatrogenic |
| Cyclophosphamide (Cytoxan), pelvic radiation therapy |
| Occupational |
| Exposure to aromatic amines (beta-naphthylamine) used in the manufacturing of chemical dyes and pharmaceuticals and in gas treatment plants |
| Manufacturing (cable, glass, paint, petroleum, tire and rubber, textile); service industry (hairdressers, painters, tar and asphalt workers, truck drivers) |
Pathology
Approximately 90 percent of epithelial bladder tumors are urothelial carcinomas; the remaining 10 percent are nonurothelial or mesenchymal in origin (Table 2).11 The World Health Organization/International Society of Urological Pathology classification of urothelial carcinomas was published in 1998 and favors the use of the term “urothelial” over “transitional” to describe epithelial tumors of the bladder.11 The pathologic classification and histologic grading and staging of bladder tumors are shown in Tables 211 and 3.12
| Epithelial neoplasms | ||
| Urothelial (transitional cell) | ||
| neoplasms (90 percent) | ||
| Papilloma | ||
| Flat | ||
| Papillary | ||
| Papillary urothelial carcinoma | ||
| Low malignant potential | ||
| Low-grade | ||
| High-grade | ||
| Invasive urothelial carcinoma | ||
| Lamina propria invasion | ||
| Muscularis propria (detrusor muscle) invasion | ||
| Nonurothelial neoplasms (9 percent) | ||
| Squamous cell carcinoma | ||
| Verrucous carcinoma | ||
| Adenocarcinoma | ||
| Clear cell | ||
| Hepatoid | ||
| Nonurachal | ||
| Signet ring cell | ||
| Urachal | ||
| Small cell carcinoma | ||
| Rare neoplasms | ||
| Basaloid squamous cell carcinoma | ||
| Carcinoid tumor | ||
| Lymphoepithelial carcinoma | ||
| Melanoma | ||
| Nonepithelial (mesenchymal) neoplasms (1 percent) | ||
| Benign | ||
| Hemangioma | ||
| Leiomyoma | ||
| Lipoma | ||
| Neurofibroma | ||
| Paraganglioma | ||
| Malignant | ||
| Angiosarcoma | ||
| Leiomyosarcoma | ||
| Malignant fibrous histiocytoma | ||
| Osteosarcoma | ||
| Rhabdomyosarcoma | ||
| Primary tumor (T) | |||
| TX: | Primary tumor cannot be assessed | ||
| T0: | No evidence of primary tumor | ||
| Ta: | Noninvasive papillary carcinoma | ||
| Tis: | Carcinoma in situ (“flat tumor”) | ||
| T1: | Tumor invades subepithelial connective tissue | ||
| T2: | Tumor invades muscle | ||
| pT2a: | Tumor invades superficial muscle (inner half) | ||
| pT2b: | Tumor invades deep muscle (outer half) | ||
| T3: | Tumor invades perivesical tissue | ||
| pT3a: | Microscopically | ||
| pT3b: | Macroscopically (extravesical mass) | ||
| T4: | Tumor invades any of the following: prostate, uterus, vagina, pelvic wall, abdominal wall | ||
| T4a: | Tumor invades prostate, uterus, vagina | ||
| T4b: | Tumor invades pelvic wall, abdominal wall | ||
| Regional lymph nodes (N) | |||
| Regional lymph nodes are those within the true pelvis; all others are distant lymph nodes | |||
| NX: | Regional lymph nodes cannot be assessed | ||
| N0: | No regional lymph node metastasis | ||
| N1: | Metastasis in a single lymph node, 2 cm or less in greatest dimension | ||
| N2: | Metastasis in single lymph node, more than 2 cm but not more than 5 cm in greatest dimension; or multiple lymph nodes, none more than 5 cm in greatest dimension | ||
| N3: | Metastasis in lymph node more than 5 cm in greatest dimension | ||
| Distant metastasis (M) | |||
| MX: | Distant metastasis cannot be assessed | ||
| M0: | No distant metastasis | ||
| M1: | Distant metastasis | ||
| Stage grouping | |||
| 0a | Ta | N0 | M0 |
| 0is | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2a | N0 | M0 |
| T2b | N0 | M0 | |
| III | T3a | N0 | M0 |
| T3b | N0 | M0 | |
| T4a | N0 | M0 | |
| IV | T4b | N0 | M0 |
| Any T | N1 | M0 | |
| Any T | N2 | M0 | |
| Any T | N3 | M0 | |
| Any T | Any N | M1 | |
Clinical Presentation
Painless hematuria is the most common presenting symptom.13 Gross blood throughout micturition is suggestive of bladder cancer. The incidence of bladder cancer in a patient with gross hematuria is 20 percent14,15 and with microscopic hematuria is 2 percent.16-18 Symptoms of bladder irritation, such as urinary frequency and urgency, more commonly occur in patients with bladder carcinoma in situ. Obstructive symptoms may be present if the tumor is located near the urethra or bladder neck. In advanced disease, patients may present with flank pain caused by ureteral obstruction, or with abdominal, pelvic, or bone pain from distant metastases (Table 41,13). Early bladder cancer is not detectable by physical examination; however, a mass may be palpable in advanced disease. A palpable kidney or pelvic mass may be present in metastatic disease.
| Hematuria (gross or microscopic) | |
| Irritative symptoms | |
| Dysuria, frequency, urge incontinence, urgency | |
| Obstructive symptoms | |
| Decreased force of stream, feeling of incomplete voiding, intermittent stream, straining | |
| Signs and symptoms of metastases or advanced disease | |
| Abdominal, bone, flank, or pelvic pain; anorexia, cachexia, or pallor; lower extremity edema; renal failure; respiratory symptoms (e.g., cough, dyspnea, hemoptysis); suprapubic palpable mass | |
Diagnosis
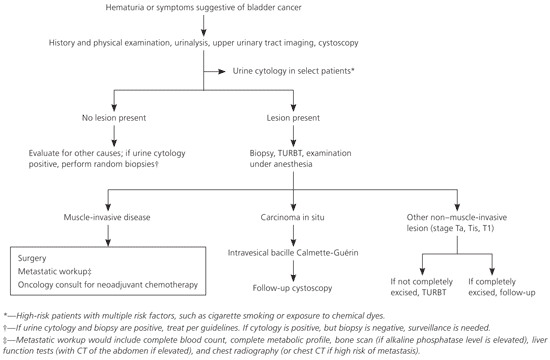
The clinical investigation should begin with a careful history, including any history of cigarette smoking or occupational exposures. Patients with urinary symptoms should have a urinalysis with urine microscopy and a urine culture to rule out infection.
URINE CYTOLOGY
Urine cytology is a noninvasive test for the diagnosis of bladder cancer. It is used to identify high-grade tumors and monitor patients for persistent or recurrent disease following treatment. Urine cytology has a high specificity (95 to 100 percent), but a low sensitivity (66 to 79 percent) for the detection of bladder cancer.19,20 This limits its usefulness for detection of bladder cancer in asymptomatic persons.
CYSTOSCOPY
Cystoscopy, an office procedure usually performed under local anesthesia, remains the mainstay of diagnosis and surveillance. Patients presenting with symptoms of bladder cancer should be evaluated with cystoscopy to determine if a lesion is present. Cystoscopy provides information about the tumor location, appearance, and size. Detection of flat neoplastic lesions, such as carcinoma in situ, can be enhanced by using fluorescence cystoscopy.18 This involves the use of a photosensitizer, such as 5-aminolevulinic acid or hexaminolevulinic acid, that is instilled intravesically. This induces macroscopic fluorescence of tumor tissue in the bladder. The procedure carries a 5 percent risk of urinary infection.
TRANSURETHRAL RESECTION OF BLADDER TUMOR
Upon detection of a lesion, a bimanual examination under anesthesia and transurethral resection of the tumor is performed. The muscle surrounding the tumor should be sampled to assess the depth of muscle invasion.
EVALUATION OF UPPER URINARY TRACT
Additional workup for all patients with bladder cancer includes evaluation of the upper urinary tract with intravenous urography (IVU), renal ultrasonography, computed tomography (CT) urography, or magnetic resonance urography.21,22 Renal ultrasonography alone is insufficient to complete the evaluation of hematuria in a patient with bladder cancer because it cannot delineate details of the urinary collecting system. Traditional IVU has been largely replaced by CT urography because of increased detail and data combined in the CT (e.g., extravesical spread of tumor, lymph node evaluation).
For patients unable to undergo contrast injection (e.g., those with allergy, renal insufficiency), magnetic resonance urography may be used to evaluate the upper urinary tract. These tests are useful for disease staging and excluding other causes of hematuria. Pelvic imaging should be performed before transurethral resection to improve staging accuracy because postoperative inflammation mimics the appearance of tumor infiltration.21 Pelvic imaging also may detect synchronous upper tract urothelial cancer, which can occur in 5 percent of patients with bladder cancer.22
EVALUATION FOR METASTATIC DISEASE
Complete blood count, blood chemistry tests (including alkaline phosphatase tests), liver function tests, chest radiography, and CT or magnetic resonance imaging of the abdomen and pelvis should be included in the metastatic workup for invasive bladder cancer.12 A bone scan may be performed if the alkaline phosphatase level is elevated or if symptoms suggesting bone metastasis are present.
TUMOR MARKERS
In recent years, there has been an intense debate about the role of urine-based tumor markers in the diagnosis and surveillance of bladder cancer. Newer tumor marker tests include the bladder tumor antigen (BTA) stat test and BTA Trak test; fluorescence in situ hybridization (FISH) analysis; ImmunoCyt test; nuclear matrix protein 22 (NMP22) test and NMP22 BladderChek test; and telomeric repeat amplification protocol.16 Tumor marker tests approved by the U.S. Food and Drug Administration, such as NMP tests and FISH analysis for chromosomal changes in cells in urine, have demonstrated a superior sensitivity to urine cytology for low-grade tumors and an equivalent sensitivity for high-grade tumors and carcinoma in situ.23 No tumor markers have the specificity of traditional urine cytology for detection of bladder cancer; therefore, tumor markers should not be used for diagnosis.23
Screening
There are no current recommendations for bladder cancer screening. The U.S. Preventive Services Task Force recommends against routine screening for bladder cancer in adults (D recommendation).24 There is no good evidence that the early treatment of this disease improves long-term health outcomes. Urine-based tests, such as urine dipstick to assess for hematuria, urine cytology, and tumor markers (e.g., BTA, NMP22), have been used as screening tools. However, because of the low prevalence of bladder cancer, the positive predictive value of these tests is low.
Treatment
| Carcinoma | Treatment |
|---|---|
| Squamous cell carcinoma | Cystectomy or radiation therapy |
| Adenocarcinoma | Cystectomy or partial cystectomy |
| Methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (Platinol) ineffective; consider fluorouracil-based therapy | |
| Small cell carcinoma | Neoadjuvant or adjuvant chemotherapy using small cell regimen |
| Local treatment (surgery or radiation therapy) | |
| Mixed histology | Follow urothelial bladder carcinoma guidelines |
| Tumor | Treatment |
|---|---|
| Low-grade Ta | Transurethral resection without intravesical chemotherapy |
| Some experts suggest a single dose of intravesical chemotherapy (not immunotherapy) within 24 hours of resection to prevent recurrence | |
| High-grade Ta | Repeat transurethral resection (if lymphovascular invasion, incomplete resection, or no muscle in the specimen), consider intravesical BCG (preferred) or mitomycin |
| Carcinoma in situ/tumor in situ | Transurethral resection followed by intravesical BCG once a week for six weeks |
| Low-grade T1 | Repeat transurethral resection followed by intravesical BCG (preferred) or mitomycin |
| High-grade T1 | Repeat transurethral resection, followed by intravesical BCG or mitomycin, or cystectomy |
| T2a or T2b (organ confined) | Radical cystectomy followed by chemotherapy in high-risk patients (e.g., those with nodal involvement, high-grade histology, transmural or vascular invasion, pathologic T3 lesion) |
| T3a or T3b | Radical cystectomy followed by adjuvant chemotherapy, consider neoadjuvant chemotherapy |
| Two trials have shown survival benefit with neoadjuvant chemotherapy (three cycles of methotrexate, vinblastine, doxorubicin [Adriamycin], and cisplatin [Platinol]) in T2 or T3 disease | |
| T4a, T4b, or metastatic disease | Chemotherapy alone or in combination with radiation therapy, except in high-risk patients (e.g., those with poor performance status, visceral [lung or liver] disease, bone disease, poor cardiac status) |
| Radiation therapy not routinely used in the United States for locally advanced bladder cancer |
| Tumor | Follow-up |
|---|---|
| Low-grade Ta | Cystoscopy every three months for one year; increase interval as appropriate |
| T1 and high-grade Ta | Cystoscopy and urine cytology every three months for two years, then every six months for two years, then annually |
| Imaging of the upper tract collecting system every one to two years | |
| Urinary tumor markers for urothelial cancer optional | |
| T2 and above (muscle-invasive disease) | Liver function test, creatinine clearance test, electrolyte panel, chest radiography every six to 12 months |
| Imaging of upper urinary tract, abdomen, and pelvis for recurrence every three to six months for two years, and then as clinically indicated | |
| If bladder-sparing surgery, urine cytology with or without biopsy every three months for one year, then increase interval | |
| If cystectomy, urine cytology every six to 12 months | |
| If cystectomy and cutaneous diversion, urethral wash cytology every six to 12 months | |
| If cystectomy and continent orthotopic diversion, monitor vitamin B12 level annually* |
TREATMENT OF NON-MUSCLE-INVASIVE DISEASE
Three stages of bladder cancer (non-muscle-invasive papillary carcinoma [stage Ta], carcinoma in situ [stage Tis], and tumor invading the lamina propria [stage T1]) were previously referred to as superficial bladder cancer, but now are described as non-muscle-invasive bladder cancer. Approximately 70 to 75 percent of bladder cancers present as non-muscle-invasive tumors. These tumors are initially treated by transurethral resection followed by close observation or intravesical chemotherapy or immunotherapy.25
Depth and grade of tumor invasion, completeness of resection, and estimated probability of recurrence are key factors that guide the use of intravesical therapy. Low-grade Ta cancers are treated with resection alone. High-grade Ta and T1 cancers have a higher risk of recurrence and progression to more invasive stages, and therefore may require further resection (repeat transurethral resection of bladder tumor), intravesical bacille Calmette-Guérin (BCG), or mitomycin. Intravesical BCG therapy is preferred over mitomycin for those at high risk of disease progression. Radical cystectomy should be considered for high-risk, non-muscle-invasive bladder cancers, such as recurrent high-grade T1 disease or tumors with micropapillary histology.27 Bladder carcinoma in situ is believed to be a precursor of invasive disease. Transurethral resection followed by intravesical BCG once per week for six weeks is recommended for carcinoma in situ.
MUSCLE-INVASIVE DISEASE
Radical cystectomy with pelvic lymphadenectomy is the standard treatment for muscle-invasive bladder cancer (stage T2 and above).28 Long-term, disease-free survival rates of up to 70 percent can be achieved if pathology shows no extravesical diseases.28 Radical cystectomy involves cystoprostatectomy in men and cystectomy (usually with hysterectomy) in women, followed by a urinary diversion procedure. Segmental cystectomy may be considered for solitary lesions without carcinoma in situ located in suitable locations. In patients with extensive comorbid disease, bladder preservation strategies (transurethral resection with or without chemotherapy) are sometimes used instead of cystectomy to limit treatment-associated morbidity.
Neoadjuvant and adjuvant chemotherapy has been explored in patients with muscle-invasive disease and is best reserved for high-risk patients (e.g., those with nodal involvement, high-grade histology, transmural or vascular invasion, pathologic T3 lesion). Combination gemcitabine (Gemzar) and cisplatin (Platinol) is the standard treatment for most patients because of its lower toxicity. Combination methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (MVAC) has comparable effectiveness to gemcitabine/cisplatin; however, toxicity limits its use.29 Chemotherapy is not used for nonurothelial cancers, such as squamous cell carcinoma or adenocarcinoma, which are primarily treated with cystectomy.
METASTATIC DISEASE
Patients with metastatic disease are treated with chemotherapy. The specific chemotherapy regimen depends on existence of medical comorbidities, such as cardiac and renal dysfunction. A commonly used combination is cisplatin and gemcitabine or a multidrug cisplatin-based regimen, such as MVAC. Carboplatin-based regimens are used in patients with insufficient renal reserve. If muscle invasion has occurred, radical cystectomy with pelvic lymphadenectomy remains the treatment of choice. Lifestyle modifications, including smoking cessation, are an integral part of treatment. Upon diagnosis, patients require continuous surveillance (Table 725,26) because most recurrences can be successfully treated if detected early.