
Am Fam Physician. 2009;80(10):1089-1094
Patient information: See related handout on supraventricular tachycardia, written by the author of this article.
Author disclosure: Nothing to disclose.
Supraventricular arrhythmias are relatively common, often persistent, and rarely life-threatening cardiac rhythm disturbances that arise from the sinus node, atrial tissue, or junctional sites between the atria and ventricles. The term “supraventricular arrhythmia” most often is used to refer to supraventricular tachycardias and atrial flutter. The term “supraventricular tachycardia” commonly refers to atrial tachycardia, atrioventricular nodal reentrant tachycardia, and atrioventricular reciprocating tachycardia, an entity that includes Wolff-Parkinson-White syndrome. Atrial fibrillation is a distinct entity classified separately. Depending on the arrhythmia, catheter ablation is a treatment option at initial diagnosis, when symptoms develop, or if medical therapy fails. Catheter ablation of supraventricular tachycardias, atrial flutter, and atrial fibrillation offers patients high effectiveness rates, durable (and often permanent) therapeutic end points, and low complication rates. Catheter ablation effectiveness rates exceed 88 percent for atrioventricular nodal reentrant tachycardia, atrioventricular reciprocating tachycardia, and atrial flutter; are greater than 86 percent for atrial tachycardia; and range from 60 to 80 percent for atrial fibrillation. Complication rates for supraventricular tachycardias and atrial flutter ablation are 0 to 8 percent. The complication rates for atrial fibrillation ablation range from 6 to 10 percent. Complications associated with catheter ablation result from radiation exposure, vascular access (e.g., hematomas, cardiac perforation with tamponade), catheter manipulation (e.g., cardiac perforation with tamponade, thromboembolic events), or ablation energy delivery (e.g., atrioventricular nodal block).
Supraventricular arrhythmias, a family of cardiac arrhythmias including supraventricular tachycardias and atrial flutter, are common, often persistent, and rarely life threatening. They arise from the sinus node, atrial tissue, or junctional sites between the atria and ventricles, and are amenable to medical and catheter-based therapies. The term “supraventricular tachycardia” commonly refers to atrial tachycardia, atrioventricular nodal reentrant tachycardia (AVNRT), and atrioventricular reciprocating tachycardia (AVRT). Atrial fibrillation is a distinct entity classified separately.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Catheter ablation is a relatively safe procedure that provides a high rate of effectiveness for most arrhythmias treated. The complication rate is highest for atrial fibrillation. | C | 1, 23 |
| Catheter ablation is first-line therapy for many supraventricular arrhythmias, including atrioventricular nodal reentrant tachycardia, symptomatic atrioventricular reciprocating tachycardia, atrial flutter, and symptomatic or incessant atrial tachycardia. | C | 1 |
| Catheter ablation of atrial fibrillation is an option in symptomatic patients with a normal left atrial size and in whom antiarrhythmic medications have failed. | C | 23, 26 |
Although antiarrhythmic medications can be used for treatment, they often lack effectiveness, are associated with multiple adverse effects, and are prone to drug-drug interactions. Thus, a principal therapy for many supraventricular arrhythmias is catheter-based ablation. Ablation can safely treat, if not cure, many common dysrhythmias, with excellent effectiveness (Table 11–26) and without incurring the long-term, sometimes morbid, consequences of antiarrhythmic drug therapy. Catheter ablation is first-line therapy for many supraventricular arrhythmias, including AVNRT, symptomatic AVRT, atrial flutter, and symptomatic or incessant atrial tachycardia.1
| Arrhythmia | Ablation location | Success rate (%) | Complication rate (%) | Potential complications | Indications |
|---|---|---|---|---|---|
| Atrial tachycardia | Variable, anywhere in right or left atrium | 86 to 1001–3 | 0 to 81 | Atrioventricular or sinus node dysfunction; cardiac perforation with tamponade; phrenic nerve injury1 | Incessant tachycardia; symptomatic tachycardia; tachycardia-mediated cardiomyopathy1 |
| Atrioventricular nodal reentrant tachycardia | Right atrium, perinodal | 961,4,5 | 0.5 to 16,7 | Atrioventricular nodal block, usually requiring pacemaker implantation6,7 | First-line therapy in patients willing and able to undergo catheter ablation1 |
| Atrioventricular reciprocating tachycardia | Variable, left or right atrioventricular ring, septal | Approximately 955,8–12 | 2 to 41,6,13 | Complete atrioventricular nodal block; cardiac perforation with tamponade1,6,13 | First-line therapy for symptoms, single or infrequent episodes, or with atrial fibrillation and rapid conduction1 |
| Atrial flutter | Right atrium, if common variety of atrial flutter | 88 to 10014–19 | 2.5 to 3.517,18,20–22 | Cardiac perforation with tamponade; heart block; myocardial infarction; thromboembolic events20–22 | First-line therapy for poorly tolerated or recurrent episodes1 |
| Atrial fibrillation | Left atrium, pulmonary vein antra | 60 to 8023,24 | 6 to 1023–25 | Cardiac perforation with tamponade; phrenic nerve injury; pulmonary vein stenosis; thromboembolic events; vascular complications1,25 | Alternative to drug therapy for recurrent atrial fibrillation in symptomatic patients with minimal or no left atrial enlargement23,26 |
Catheter-based ablation of a cardiac arrhythmia is performed in an electrophysiology laboratory in conjunction with an electrophysiology study. An electrophysiology study involves the percutaneous insertion of catheters into the femoral veins and often the internal jugular vein. The catheter tips are positioned at specific locations in the heart. Electrical stimulation is delivered to the myocardium via these catheters to characterize cardiac conduction and arrhythmias. Once the patient's conduction system has been studied and the rhythm disturbance diagnosed, an ablation catheter is used to thermally destroy the pathogenic myocardial tissue underlying the arrhythmia's initiation or maintenance. Success and complication rates vary, depending on the individual arrhythmia (Table 11–26). Introduction of catheters into the heart, with or without the delivery of ablative energy, uniformly carries the risk of cardiac perforation and possibly tamponade. If detected early and in the absence of systemic anticoagulation, iatrogenic cardiac tamponade caused by catheter perforation uncommonly is a life-threatening complication; however, it does require the percutaneous insertion of a temporary subxiphoid pericardial drain if associated with hemodynamic compromise.
Atrial Tachycardia
Sustained atrial tachycardia is a relatively uncommon arrhythmia diagnosed in about 5 to 15 percent of patients referred for supraventricular tachycardia ablation, but with increasing age, it constitutes a larger percentage of supraventricular tachycardias.27 It is a focal arrhythmia that can arise from anywhere in the right or left atrium. For atrial tachycardia ablation, success rates are 86 to 100 percent, with a recurrence rate of 0 to 8 percent.1–3 Uncommon complications (0 to 8 percent) include cardiac perforation, phrenic nerve injury, and atrioventricular or sinus node dysfunction.1 Catheter ablation of atrial tachycardia is reserved for symptomatic cases refractory to medical therapy and for patients who have developed a tachycardia-mediated cardiomyopathy because of prolonged exposure to rapid heart rates.1
Atrioventricular Nodal Reentrant Tachycardia
AVNRT is the most common supraventricular tachycardia referred for treatment by catheter ablation28 and demonstrates a 2:1 predominance in women.27 Catheter ablation of AVNRT is successful in approximately 96 percent of cases, with recurrence rates of 3 to 7 percent.1,4–6,29–31 The principal potential complication related to AVNRT ablation is atrioventricular nodal block (0.5 to 1 percent); palpitations and inappropriate sinus tachycardia also have been described postablation.6,7 Ablation is recommended as first-line therapy in most cases of AVNRT, but must be tailored to the individual patient's lifestyle and concomitant medical conditions.1
Atrioventricular Reciprocating Tachycardia
AVRT, a category of supraventricular tachycardias under which Wolff-Parkinson-White syndrome falls, involves the transmission of electrical impulses across one or more extranodal (accessory) pathways. Accessory pathway electrical conduction can be manifested on electrocardiography (ECG) as a slurring (delta wave) of the initial portion of the QRS complex. Accessory pathway conduction also can be present without apparent delta wave, in which case it is referred to as a concealed pathway. The effectiveness of catheter ablation of accessory pathways is approximately 95 percent in most series,5,8–12 with recurrence rates of approximately 5 percent.1 The overall complication rate is 2 to 4 percent, with the most common complications being complete atrioventricular nodal block and cardiac perforation with tamponade.1,6,13 The presence of manifest accessory pathway conduction (delta wave on the surface ECG) in association with tachycardia symptoms or documented tachycardia should prompt referral for catheter ablation.1
Atrial Flutter
Atrial flutter constitutes approximately 15 percent of all supraventricular arrhythmias and occurs in 25 to 35 percent of patients with atrial fibrillation.1 Atrial flutter usually is more symptomatic than atrial fibrillation because it is often associated with more rapid ventricular rates. The electrical circuit causing the most common forms of atrial flutter is anatomically well defined and can be interrupted readily with ablation near the junction of the inferior vena cava and the right atrium. Long-term success rates for ablation of typical forms of atrial flutter range from 88 to 100 percent,14–19 and patients treated with ablation have lower hospitalization rates than patients treated with antiarrhythmic drugs.32 Complications from ablation occur at a rate of 2.5 to 3.5 percent and include heart block, cardiac perforation with tamponade, thromboembolic events, and myocardial infarction.17,18,20–22 Catheter ablation is recommended in most cases of atrial flutter.1
Atrial Fibrillation
Atrial fibrillation is the most common clinically significant arrhythmia, with an estimated prevalence of 0.4 to 1.0 percent in the general population.26 Atrial fibrillation is associated with an increased risk of stroke, heart failure, and all-cause mortality.26 Management principles focus on adequate anticoagulation (to prevent embolic stroke occurrence), ventricular rate control measures (to prevent symptomatic and pathogenic tachycardia), and in selected patients, rhythm control strategies (to restore and maintain sinus rhythm).
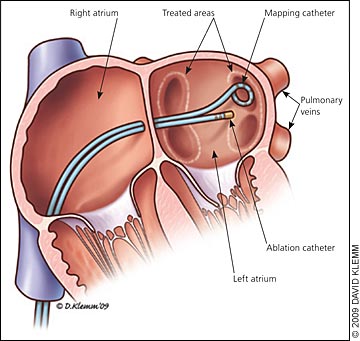
Atrial fibrillation ablation seeks to establish and maintain sinus rhythm by targeting the tissue interface between the pulmonary veins and the left atrium, an anatomic region that plays a critical role in initiating and perpetuating atrial fibrillation33–43 (Figure 1). Patients who are most likely to benefit from atrial fibrillation ablation are those with a normal left atrial size and in whom atrial fibrillation is symptomatic and refractory to one or more antiarrhythmic medications.23 Symptoms factor heavily in determining whether atrial fibrillation ablation is worthwhile, because quality-of-life scores demonstrate improvement after ablative therapy.23,26,44,45 As such, ablation of atrial fibrillation is undertaken as an alternative to pharmacologic treatment to prevent recurrent atrial fibrillation in symptomatic patients with minimal or no left atrial enlargement.26 A recent meta-analysis found that radiofrequency ablation after failed antiarrhythmic drug therapy maintained sinus rhythm more than continuation of drug therapy alone.46
Overall success rates for the elimination of atrial fibrillation via catheter ablation vary depending on the data examined, patient clinical factors, the number of atrial fibrillation ablation procedures the patient has undergone, as well as the definition of a successful ablation. Paroxysmal atrial fibrillation has higher elimination rates at one year (roughly 75 to 80 percent) than persistent atrial fibrillation (60 to 70 percent), with repeat ablations for atrial fibrillation resulting in higher effectiveness rates.23,24 These differing ablation success rates come from the observation that the longer atrial fibrillation is present, the more the left atrium histologically is altered to promote and perpetuate atrial fibrillation. The risk of a major complication with an atrial fibrillation ablation procedure is approximately 6 percent.23–25 The most common major complication is cardiac tamponade,1,25 and the most common minor complication is groin hematoma formation at catheter insertion sites (13 percent).1
Patient Experience
Typically, a patient will present to his or her family physician with palpitations. An externally worn, ambulatory ECG monitor commonly is used to correlate symptoms of palpitations with a specific supraventricular arrhythmia. These monitors can be worn for up to a month. If an ambulatory monitor fails to identify a specific arrhythmia, an implantable monitor can be inserted surgically to screen for arrhythmias for up to several years. If and when a supraventricular arrhythmia is identified, the patient is usually referred to a cardiologist, and subsequently, an electrophysiologist. Ultimately, a decision is made regarding pharmacologic rhythm control versus an electrophysiology study and ablation.
If the patient elects for an electrophysiology study and ablation, he or she should fast after midnight the night before the procedure and not take medications that may interfere with tachycardia inducibility at the electrophysiology study. On arrival to the electrophysiology laboratory, routine preoperative care occurs, with insertion of an intravenous line and often, assessment by an anesthesiologist. Analgesia protocols range from conscious sedation to general endotracheal anesthesia. The former is used when excess sedation threatens to render the clinical arrhythmia quiescent, and thus, not ablatable. Heavier sedation may be needed for complex, protracted ablations in which arrhythmia induction is not required or when patient immobility is important.
During the procedure, the patient lies flat for several hours; the exact length of time is dependent on the complexity of the arrhythmia. AVNRT ablations tend to be shorter (less than three hours) than atrial fibrillation ablations (which can last four to eight hours). During and after the procedure, patients may have back pain and anxiety. Chest pressure or pleuritic pain may be experienced during the delivery of ablation energy (a relatively small percentage of the total procedural time). On completion of the procedure, the catheters and sheaths are removed from their insertion sites, and manual pressure is applied to achieve hemostasis. Typically, the patient is discharged later the same day or the next day. Full recovery typically takes two or three days, with longer recuperation times for more complex procedures.
Radiation Exposure
As with cardiac catheterization and angioplasty, fluoroscopy is the principal means of visualizing and modifying catheter position during an electrophysiology study and arrhythmia ablation. The duration of fluoroscopy (and thus, the degree of radiation exposure) varies depending on the type and sophistication of the ablation procedure (Table 2).47–53 The measurement for radiation doses used in medical procedures, including an electro-physiology study and ablation, is the millisievert (mSv). Millisieverts can be converted into chest radiography equivalent doses, which is the dose of radiation that is received from a single chest radiograph. One chest radiography equivalent dose is 0.1 mSv.47 The radiation doses for an electrophysiology study and ablation are estimated to be 3.2 mSv (chest radiography equivalent dose = 32) and 15.2 mSv (chest radiography equivalent dose = 152), respectively,48 with a range of 1.4 mSv (chest radiography equivalent dose = 14) to 49.75 mSv (chest radiography equivalent dose = 497) for combined electrophysiology study and ablation reported in several studies.48–52
The potential long-term risks of radiation exposure that accompany catheter ablation procedures are rare and include skin injury,54 malignancy, and teratogenicity. Overall risk of fatal malignancy caused by radiation from an electrophysiology study with ablation was found to be 0.03 percent for 60 minutes of fluoroscopy, and the risk of teratogenicity was found to be one to 20 per 1 million births per 60 minutes of fluoroscopy.49,55 Radiation exposure can be limited by the implementation of adjunctive three-dimensional computer mapping software that creates a real-time virtual map of the anatomic area of interest, as well as ablation catheter position.
| Study or procedure | Average dose (mSv) | Chest radiography equivalent dose |
|---|---|---|
| Chest radiography | 0.147 | 1 |
| Electrophysiology study and ablation | 3.2 (electrophysiology study) and 15.2 (ablation), with a range of 1.4 to 49.75 for combined electrophysiology study and ablation48–52 | 32 (electrophysiology study) and 152 (ablation), with a range of 14 to 497 for combined electrophysiology study and ablation |
| Atrial tachycardia ablation | 4.4 (1.7 to 7.2)48–52 | 44 (17 to 72) |
| Atrioventricular nodal reentrant tachycardia ablation | 4.8 (0.6 to 29.9)48–52 | 48 (6 to 299) |
| Computed tomography of chest | 847 | 80 |
| Atrial flutter ablation | 12.1 (4.7 to 28.4)48–52 | 121 (47 to 284) |
| Atrioventricular reciprocating tachycardia ablation | 12.8 (1.1 to 35.1)48–52 | 128 (11 to 35) |
| Coronary angiography with stent placement | 1353 | 130 |
| Atrial fibrillation ablation | 16.6 (10.1 to 23.0)48–52 | 166 (101 to 230) |