
Am Fam Physician. 2009;80(11):1245-1251
Patient information: See related handout on coronary artery disease and the use of stents, written by the authors of this article.
Author disclosure: Nothing to disclose.
Many advances have been made in the percutaneous treatment of coronary artery disease during the past 30 years. Although balloon angioplasty alone is still performed, the use of coronary artery stents is much more common. Approximately 40 percent of patients treated with balloon angioplasty developed restenosis, and this was reduced to roughly 30 percent with the use of bare-metal stents. However, restenosis within the stent can occur and is difficult to treat. Drug-eluting stents were developed to lower the rate of restenosis, which now occurs in less than 10 percent of patients treated with these stents. There have been concerns about abrupt thrombosis within drug-eluting stents occurring late after their implantation, leading to acute myocardial infarction and death. Recent studies have alleviated, but not completely dispelled, these concerns. Strict adherence to dual antiplatelet therapy with aspirin and a thienopyridine is required after stent placement, and the premature discontinuation of therapy is the most important risk factor for acute stent thrombosis. Adequate communication between cardiologists and primary care physicians is essential not only to avoid the premature discontinuation of therapy, but also to identify, before stent placement, those patients in whom prolonged antiplatelet therapy may be ill-advised. Elective surgery following stent placement should be delayed until the recommended course of dual antiplatelet therapy has been completed.
Since the first balloon angioplasty, the use of percutaneous coronary intervention has grown substantially. Advances in this technique have expanded the indications for the procedure, dramatically improved safety, and reduced the rate of restenosis1,2 (Table 12–4). Many factors contributed to the growth of percutaneous coronary intervention, but the development of coronary artery stents was the pivotal event. This article focuses on the types of stents used, indications for their use, potential complications, and recommendations for drug therapy after stent placement.
| Type of intervention | Restenosis rate (%) | Mortality rate of procedure (%) | Emergency CABG rate (%) |
|---|---|---|---|
| Balloon angioplasty | 40 | 1 to 2.5 | 1.9 to 5.8 |
| Bare-metal stent | 30 | < 0.25 | < 0.3 to 0.6 |
| Drug-eluting stent | < 10 | < 0.25 | < 0.3 to 0.6 |
Balloon Angioplasty and Stenting
A major limitation of balloon angioplasty is lesion restenosis, which occurs in up to 40 percent of treated patients.5 Coronary stents were developed to combat some of the biologic processes that lead to restenosis. These stents are metal scaffolds of various designs that are compressed on the outside of the balloon catheter. The stent is positioned across the stenosis and the balloon is then inflated, expanding the stent into the vessel wall. After deflation and removal of the balloon, the stent remains in the artery, minimizing the progressive constriction (restenosis) of the treated lesion. Bare-metal stents reduced the occurrence of restenosis to roughly 30 percent, compared with 40 percent for balloon angioplasty alone.3 In addition, stents nearly eliminated the likelihood of severe coronary artery dissections, markedly reducing the need for emergency bypass surgery.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The recommended dosages for dual antiplatelet therapy are 162 to 325 mg of aspirin daily and 75 mg of clopidogrel (Plavix) or 10 mg of prasugrel (Effient) daily, but ticlopidine (formerly Ticlid) in a dosage of 250 mg twice daily may be used if the patient cannot tolerate clopidogrel or prasugrel. | C | 14, 19, 30, 31 |
| The preferred duration of dual antiplatelet therapy after bare-metal or drug-eluting stent placement is one year. | C | 19, 30, 31 |
| The minimum recommended duration of dual antiplatelet therapy after stent placement is one month for bare-metal stents, three months for the sirolimus (Rapamune)-eluting stent (Cypher), and six months for other drug-eluting stents. In special circumstances, two weeks of therapy after bare-metal stent placement may be considered. | C | 11, 14, 19, 30,31 |
| Patients at increased risk of gastrointestinal bleeding should receive acid suppression therapy while receiving dual antiplatelet therapy. Proton pump inhibitors may interfere with clopidogrel metabolism, but data are conflicting so an H2-receptor blocker or antacids may be preferable. | C | 32–35 |
| After the recommended duration of treatment with dual antiplatelet therapy, aspirin (75 to 162 mg daily) should be continued indefinitely. | C | 19, 31 |
| Following stent placement, elective surgery should be delayed until the recommended course of dual antiplatelet therapy is completed. If surgery cannot be delayed, it should be performed while the patient is on dual antiplatelet therapy. If that is not feasible, the thienopyridine should be stopped for the shortest time possible and then restarted. | C | 19, 32, 39 |
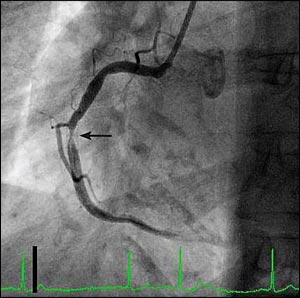
Although bare-metal stents effectively address two of the mechanisms leading to restenosis (i.e., elastic recoil and vessel constriction during healing), they do not prevent intimal and smooth muscle hyperplasia. Rather, they cause an exaggerated proliferative response, but this usually does not cause restenosis because the vessel lumen is considerably larger after stent placement compared with balloon angioplasty alone.6 However, when restenosis occurs within a bare-metal stent, it can be difficult to treat and has a very high recurrence rate7 (Figure 1). Drug-eluting stents were developed to minimize the enhanced proliferative response and further reduce the incidence of restenosis after percutaneous coronary intervention.4
Drug-eluting stents are coated with a drug delivery system that allows the controlled release of the drug over time, usually the first 30 to 45 days after implantation, which is the period of greatest tissue proliferation. Drug-eluting stents have further reduced the occurrence of early restenosis to less than 10 percent in most lesions, although it is higher in more complex lesions.8 Four drug-eluting stents are currently available, differing in the type of metal used, stent design, and drug coating (Table 2).
| Stent trade name | Metal platform | Drug used |
|---|---|---|
| Cypher | Stainless steel | Sirolimus (Rapamune) |
| Endeavor | Cobalt-chromium | Zotarolimus |
| Taxus | Stainless steel | Paclitaxel (Taxol) |
| Xience, Promus | Cobalt-chromium | Everolimus (Afinitor) |
Choosing a Stent
Balloon angioplasty is now rarely used alone, although it is often used to dilate the stenosis before stent placement. Two main factors are considered when choosing between a bare-metal or a drug-eluting stent. First, the presence of clinical or technical factors associated with an increased risk of restenosis is considered; such patients benefit most from a drug-eluting stent. Multiple randomized trials have shown that drug-eluting stents provide better long-term outcomes than bare-metal stents in the following populations: patients with diabetes,9 long and/or complex stenoses,10 acute myocardial infarction (MI),11 in-stent restenosis of a bare-metal stent,12 and total coronary artery occlusions.13 The risk of restenosis in a large coronary artery (3.5 to 4.0 mm in diameter) with a focal stenosis is sufficiently low that the incremental benefit of a drug-eluting stent is small compared with a bare-metal stent.
Second, the patient's ability to comply with the necessary dual antiplatelet therapy is carefully considered. Patients with a history of poor compliance, an increased risk of bleeding, or those who are known to need surgery or invasive procedures that would necessitate the premature withdrawal of antiplatelet therapy are often better served by placement of a bare-metal stent because the duration of dual antiplatelet therapy is shorter. However, depending on the individual circumstances, surgical revascularization or further increasing the intensity of medical therapy may be appropriate.
Stent Thrombosis
Acute stent thrombosis has been a concern since bare-metal stents were first introduced into clinical practice. Ensuring that the stent has adequately expanded to its full size within the vessel and completely apposed to the arterial wall is critical. Because it has fewer side effects, clopidogrel (Plavix) has replaced ticlopidine (formerly Ticlid), which, in combination with aspirin and optimal stent implantation methods, has reduced the occurrence of early stent thrombosis.14 A new antiplatelet agent, prasugrel (Effient), was recently approved by the U.S. Food and Drug Administration and is now being used in selected patients.
In late 2006, however, several analyses of long-term follow-up data from the initial drug-eluting stent trials plus registry data suggested an increased rate of late stent thrombosis occurring more than one year after stent placement.15 Late cardiac death and MI occurred in 4.9 percent of patients with drug-eluting stents, compared with 1.3 percent of patients with bare-metal stents between seven and 18 months following the discontinuation of clopidogrel.16 Although stent thrombosis after placement of a drug-eluting stent remained an uncommon event, occurring in approximately 0.5 to 1.0 percent of patients,17,18 the resulting acute ST-segment elevation MI had a mortality rate as high as 45 percent in some reports18 (Figure 2).
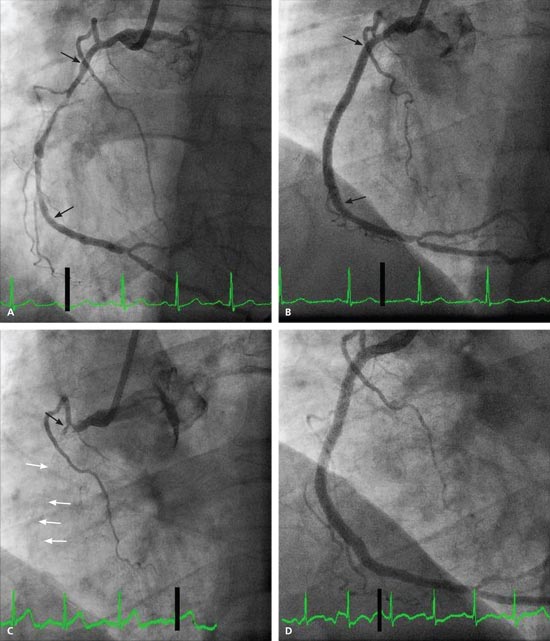
Because of these concerns, use of drug-eluting stents declined sharply, and there were some initial efforts in the United Kingdom to remove them from use altogether.19 This concern also stimulated more studies with even longer follow-up to more carefully evaluate stent thrombosis using a standard definition. These new and updated data showed that, compared with bare-metal stents, there was no increased risk of death or MI associated with drug-eluting stents.20-23 However, there was a slight difference in the timing of certain events, which basically cancel each other out over time.
Initially, restenosis after bare-metal stent placement was thought to present almost exclusively as recurrent angina that often required another revascularization procedure. However, it is now known that about 10 percent of patients with bare-metal stent restenosis present with an acute MI that can be fatal.24 Although the percentage is small, it is important to consider the morbidity and mortality of the additional revascularization procedure—a procedure that likely would have been unnecessary had a drug-eluting stent been used initially. These risks are balanced against a slight increase in the rate of late stent thrombosis, leading to death and MI with drug-eluting stents.
Causes of Stent Thrombosis
The agents used in drug-eluting stents inhibit intimal and smooth muscle growth after stent placement, but also inhibit the normal healing process whereby the stent is eventually covered by healthy endothelium. This leaves metal and polymer exposed to the blood, which can stimulate late thrombus formation after antiplatelet therapy is stopped. Furthermore, for reasons not completely understood, a small number of patients treated with drug-eluting stents have increased cell death (apoptosis) in the artery wall behind the stent, resulting in small pockets in which blood stasis and thrombosis can occur.25
Currently, there is no way to predict which patients have incomplete endothelialization or increased apoptosis after drug-eluting stent placement. Although these technical factors are important, the major factor contributing to stent thrombosis is the premature discontinuation of dual antiplatelet therapy.18 About one in seven patients who receive a drug-eluting stent discontinues antiplatelet therapy within 30 days of percutaneous coronary intervention.26
On-Label vs. Off-Label Use
The initial studies showing the reduction in restenosis with drug-eluting stents included patients and lesions at low to moderate risk of restenosis, while excluding high-risk patient groups and the complex lesions often encountered in everyday practice. When released for clinical use, drug-eluting stents were approved only for patients with lesion characteristics studied in the initial trials (“on-label” uses), but were also used in patients and lesion subsets not included in those trials (“off-label” uses).
In general, the results of drug-eluting stents in off-label uses are less favorable than in on-label uses, but they are substantially better than those of bare-metal stents for the same off-label uses.27-29 Stent thrombosis is also slightly higher in off-label use, regardless of whether a bare-metal or drug-eluting stent is used.27
Postprocedural Medical Therapy
Following placement of a stent, strict adherence to dual antiplatelet therapy with aspirin, 162 to 325 mg daily, and a thienopyridine derivative is imperative.14,19,30,31 Clopidogrel, 75 mg daily, is preferred because of fewer side effects, but ticlopidine, 250 mg twice daily, may be substituted if clopidogrel is not tolerated. Prasugrel, 10 mg daily, is now being substituted for clopidogrel in patients with a higher risk of stent thrombosis, but it also imparts a higher risk of bleeding. The optimal duration of dual antiplatelet therapy following drug-eluting stent placement is unknown.
When drug-eluting stents were initially released for clinical use, the duration of antiplatelet therapy reflected what was used in the pivotal trials leading to their approval. However, when concerns over late stent thrombosis emerged, several professional organizations published recommendations that dual antiplatelet therapy be extended for one year in patients not at risk for bleeding30,31 (Table 330). Although one year of dual anti-platelet therapy following stent placement is optimal, the minimum recommended duration after placement is one month for bare-metal stents, three months for sirolimus (Rapamune)-eluting stents, and six months for other drug-eluting stents.11,14,19,30,31 Patients must be strictly instructed to not discontinue their antiplatelet medications without first consulting their cardiologist.
| 1. Before stent implantation, the physician should discuss the necessity of dual antiplatelet therapy. In patients not expected to comply with 12 months of thienopyridine therapy, for economic or other reasons, strong consideration should be given to avoiding a drug-eluting stent. |
| 2. In patients preparing for percutaneous coronary intervention who are likely to require invasive or surgical procedures within the next 12 months, consideration should be given to use of a bare-metal stent or balloon angioplasty with provisional stent implantation instead of the routine use of a drug-eluting stent. |
| 3. A greater effort by health care professionals must be made before patient discharge to ensure that patients are properly and thoroughly educated about the reasons for which they are prescribed thienopyridines and the significant risks associated with prematurely discontinuing such therapy. |
| 4. Patients should be specifically instructed before hospital discharge to contact their treating cardiologist before stopping any antiplatelet therapy, even if told to stop such therapy by another health care professional. |
| 5. Health care professionals who perform invasive or surgical procedures and are concerned about peri- and postprocedural bleeding must be made aware of the potentially catastrophic risks of premature discontinuation of thienopyridine therapy. These professionals should contact the patient's cardiologist if issues about the patient's antiplatelet therapy are unclear, to discuss optimal patient management strategy. |
| 6. Elective procedures for which there is significant risk of peri- or postoperative bleeding should be deferred until patients have completed an appropriate course of thienopyridine therapy (12 months after drug-eluting stent implantation if they are not at high risk of bleeding, and a minimum of one month for bare-metal stent implantation). |
| 7. For patients with drug-eluting stents who will have subsequent procedures that mandate discontinuation of thienopyridine therapy, aspirin should be continued if at all possible and the thienopyridine restarted as soon as possible after the procedure because of concerns about late stent thrombosis. |
| 8. The health care industry, insurers, the U.S. Congress, and the pharmaceutical industry should ensure that issues such as drug cost do not cause patients to prematurely discontinue thienopyridine therapy, which could cause catastrophic cardiovascular complications. |
In those at increased risk of gastrointestinal bleeding, the addition of a proton pump inhibitor has been recommended.32 Conflicting data exist about the ability of certain proton pump inhibitors to interfere with the metabolism of clopidogrel.33-35 Until this is clarified, no firm recommendation can be made, but substitution of an histamine H2-receptor blocker or antacids may be an alternative. After the recommended duration of treatment with dual antiplatelet therapy, aspirin (75 to 162 mg daily) should be continued indefinitely.19,31 Family physicians have an important role in emphasizing the need for dual antiplatelet therapy and monitoring the use of other medications as they help coordinate future care.
Elective or Emergency Surgery After Stent Placement
Patients or their family physicians may know of the need for future invasive procedures or surgery when the patient presents with a clinical situation requiring stent placement. This information must be disclosed to the cardiologist before the procedure, because it will affect the choice of stent used. Based on expert opinion, elective surgery should not be performed within one month following bare-metal stent placement, but may be considered after two weeks in special circumstances. Elective surgery should be delayed and dual antiplate-let therapy continued for at least one year after drug-eluting stent placement (Table 4).36 Once these periods have elapsed, elective surgery may be performed with the patient receiving aspirin alone, but this does not provide a guarantee that stent thrombosis will be avoided.37
| Type of intervention | Timing of elective surgery following intervention |
|---|---|
| Balloon angioplasty | After at least two weeks |
| Bare-metal stent placement | After one month recommended, but minimum of two weeks in special circumstances |
| Drug-eluting stent placement | After one year |
Data on performing elective noncardiac surgery in patients taking dual antiplatelet therapy are limited, but continuing therapy through procedures with a low bleeding risk (i.e., dental,38 dermatologic, or ophthalmologic) may be considered. If elective surgery cannot be delayed, the possibility of continuing aspirin and a thienopyridine should be discussed with the surgeon, but there is often considerable reluctance to operate on patients receiving these drugs. A recent review of stent thrombosis cases suggests that withholding clopidogrel while maintaining aspirin may be relatively safe in this setting.39 If the procedure is an emergency, measures such as local administration of thrombogenic factors and planning for blood transfusions can be considered in managing the anticipated excess bleeding. If clopidogrel must be stopped, it should be restarted as soon as possible following the procedure. Currently, no recommendations support the use of “bridging therapy” with low-molecular-weight heparin or glycoprotein IIb/IIIa inhibitors in this situation.40