Am Fam Physician. 2010;81(3):289-296
A more recent article on stable coronary artery disease is available.
Author disclosure: Nothing to disclose.
Coronary artery disease is the leading cause of mortality in the United States. In patients who have had a myocardial infarction or revascularization procedure, secondary prevention of coronary artery disease by comprehensive risk factor modification reduces mortality, decreases subsequent cardiac events, and improves quality of life. Options for secondary prevention include medical therapy and surgical revascularization in the form of coronary artery bypass grafting or percutaneous coronary intervention. Medical therapy focuses on comprehensive risk factor modification. Therapeutic lifestyle changes (including weight management, physical activity, tobacco cessation, and dietary modification) improve cardiac risk factors and are universally recommended by evidence-based guidelines. Treatment of hypertension and dyslipidemia reduces morbidity and mortality. Recommendations for persons with diabetes mellitus generally encourage glucose control, but current evidence has not shown reductions in mortality with intensive glucose management. Aspirin, angiotensin-converting enzyme inhibitors, and beta blockers reduce recurrent cardiac events in patients after myocardial infarction. Surgical revascularization by coronary artery bypass grafting is recommended for those with significant left main coronary artery stenosis, significant stenosis of the proximal left anterior descending artery, multivessel coronary disease, or disabling angina. Percutaneous coronary intervention may be considered in select patients with objective evidence of ischemia demonstrated by noninvasive testing.
Coronary artery disease (CAD) is the leading cause of death in the United States, with more than 1 million new and recurrent cardiovascular events occurring each year, and its prevalence and impact are expected to grow.1,2 Advances in treatment have improved survival after the initial event, but persons with established CAD have a high risk of future cardiovascular events.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Exercise-based cardiac rehabilitation reduces morbidity and mortality in patients with CAD. | A | 3–7 |
| Weight management is recommended by the AHA for the secondary prevention of CAD. | C | 2, 4, 11 |
| Smoking cessation reduces mortality by at least one third in patients after MI or cardiac surgery. | A | 13, 14 |
| The AHA and The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommend treating hypertension for a blood pressure goal of < 140/90 mm Hg, or < 130/80 mm Hg in patients with diabetes mellitus or chronic kidney disease. | B | 17, 18 |
| Beta-blocker therapy reduces recurrent MI, sudden cardiac death, and mortality in patients after MI. | A | 19–22 |
| Aspirin therapy (81 to 162 mg daily) reduces recurrent vascular events by one fourth in patients with a previous vascular event. | A | 2, 4, 35 |
| Statins reduce recurrent vascular events and all-cause mortality in patients following acute coronary syndromes. | A | 37, 38, 40, 41 |
| Percutaneous coronary interventions have not been shown to be superior to optimal medical treatment alone for death or recurrent cardiovascular events in patients with stable CAD. | B | 36, 52, 53 |
Recent clinical studies show that persons with CAD can reduce their risk of subsequent cardiovascular events through effective secondary prevention, which reduces mortality and improves quality of life.2 Family physicians play an important role in initiating and maintaining risk factor modification using evidence-based standards. This article reviews the risk factors for recurrent CAD, current evidence-based interventions, and comprehensive risk factor improvement strategies.
Physical Activity
Regular physical activity is an important component of secondary prevention of CAD; it increases exercise capacity, treats comorbid risk factors, and improves quality of life.3,4 Exercise-based cardiac rehabilitation has been shown to reduce all-cause and cardiac mortality compared with usual care.3–7 The goal for all patients is 30 to 60 minutes of moderate-intensity physical activity (e.g., brisk walking, biking) on most, if not all, days of the week.2–4,8 Consistent physical activity improves cardiovascular risk factors—especially total cholesterol and triglyceride levels—and systolic blood pressure.5
Exercise-based cardiac rehabilitation programs may be initiated shortly after an acute coronary syndrome or revascularization procedure.2,3 Hospital-based cardiac rehabilitation has not been shown to be superior to home-based cardiac rehabilitation for low-risk patients.9 Before patients begin a rigorous exercise program, physicians should assess their cardiovascular status by taking a physical activity history or performing an exercise test.4 Details of assessment tools and exercise prescriptions were reviewed in a previous article in American Family Physician (AFP; https://www.aafp.org/afp/2008/0415/p1129.html).10
Weight and Dietary Management
Obesity is associated with increased CAD mortality and adversely affects cardiac function and comorbid CAD risk factors.11 Obesity is classified using the body mass index (BMI; Table 1).11 Weight loss is indicated for patients who are classified as overweight or obese according to their BMI. The American Heart Association (AHA) recommends measuring BMI at each office visit, then providing objective feedback and consistent counseling on weight loss strategies.2,4,8,11 Long-term weight maintenance is best achieved through a balance of physical activity and moderation of caloric intake; improvements in cardiac risk factors are commonly observed with even modest weight loss (i.e., 10 percent of baseline weight).8,11 Insufficient evidence exists to determine whether weight reduction decreases cardiovascular mortality in persons who are obese.11 The evidence for current dietary recommendations for primary and secondary prevention of CAD is summarized in a previous article in AFP (https://www.aafp.org/afp/2009/0401/p571.html).12
| Classification | Body mass index (kg per m2) |
|---|---|
| Underweight | < 18.5 |
| Normal | 18.5 to 24.9 |
| Overweight | 25.0 to 29.9 |
| Obese | ≥ 30.0 |
Tobacco Cessation
Tobacco cessation has been shown to reduce all-cause mortality in patients with established CAD.13,14 In a recent Cochrane review, investigators concluded that persons who quit smoking after a myocardial infarction (MI) or cardiac surgery reduce their risk of death by at least one third, and that discontinuing smoking is at least as beneficial as modifying other risk factors.13,14
Physicians are encouraged to ask about tobacco use at each office visit, and to extend a clear recommendation to quit to every patient who smokes. If a patient is willing to try to quit, physicians can assist with cessation through counseling and pharmacotherapy, which are most effective when combined.15,16 Providing behavior therapy, telephone support, and self-help materials for at least one month can help patients with CAD to quit smoking.15,16
Hypertension
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and the AHA recommend treating hypertension (i.e., blood pressure greater than 140/90 mm Hg, or greater than 130/80 mm Hg for persons with diabetes mellitus or chronic kidney disease) for the secondary prevention of CAD.17,18 Lifestyle modifications involve weight management, regular physical activity, prudent alcohol consumption, and a low-sodium diet. The JNC 7 and the AHA recommend initial treatment of hypertension after an MI with beta blockers or angiotensin-converting enzyme (ACE) inhibitors, with additional medications added in a stepwise fashion to achieve goal blood pressure.17,18
Beta Blockers
Multiple clinical trials have shown that beta-blocker therapy can reduce recurrent MI, sudden cardiac death, and mortality in patients after MI, even in those who are normotensive.19–22 Consequently, the AHA has recommended that a beta-blocker regimen be initiated and maintained indefinitely for the secondary prevention of CAD in all patients after having an MI, unless contra-indicated.2,23 Common contraindications and precautions for beta-blocker therapy are listed in Table 2.22 There is no clear consensus as to which beta blocker is the safest or most effective.22
| Contraindications |
| Asthma that requires the use of bronchodilators and/or steroids |
| Cardiogenic shock |
| Heart rate < 50 to 60 beats per minute |
| Second- or third-degree atrioventricular block |
| Severe heart failure that requires the use of intravenous diuretics or inotropes |
| Systolic blood pressure < 90 to 100 mm Hg |
| Precautions |
| Chronic obstructive pulmonary disease |
| Diabetes mellitus (although some experts do not consider this a precaution) |
| First-degree atrioventricular block |
| Heart failure* |
| Peripheral vascular disease |
ACE Inhibitors
Two large randomized trials have demonstrated the benefits of ACE inhibitors in the secondary prevention of CAD. The Heart Outcomes Prevention Evaluation (HOPE) study showed that 10 mg per day of ramipril (Altace) reduced cardiovascular death and MI in those who were at high risk of or had established vascular disease without heart failure.24 The European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease (EUROPA) revealed a 20 percent reduction in cardiovascular mortality and MI in patients with stable CAD without heart failure who were treated with perindopril (Aceon).25 Investigators who performed a combined analysis of several studies concluded that there is strong evidence for consistent cardiovascular protection with ACE-inhibitor therapy by improving survival and reducing the risk of major cardiovascular events in patients with vascular disease.26
Management of Patients with Diabetes
The mortality rate of CAD is higher in patients with diabetes than in those without diabetes.27 Controversy exists regarding appropriate glucose control for diabetes management. Several guidelines recommend treatment to reduce A1C levels to less than 7 percent; however, recent randomized clinical trials have not demonstrated reductions in cardiovascular events or mortality with intensive glucose control.2,27–30 Studies have shown inconsistent improvement with intensive glucose control in microvascular complications, including nephropathy, but increased adverse effects were observed, including weight gain, fluid retention, and symptomatic hypoglycemia.28–31 The largest recent trial investigating cardiovascular outcomes with intensive glucose control was discontinued early because of a 22 percent increased risk of all-cause mortality in the group treated toward an A1C goal of 6 percent compared with less-intensive glucose control.28 In summary, recent randomized clinical trials have not shown significant reductions in cardiovascular events or mortality with intensive glucose control.28–31
Secondary prevention of CAD in patients with diabetes also includes treatment of comorbid hypertension, dyslipidemia, and hypercoagulability.32 Treatment of diabetes with statins reduces vascular morbidity and mortality regardless of cholesterol values, and a 2008 meta-analysis33 reported a proportional reduction in major vascular events, with a reduction in low-density lipoprotein (LDL) cholesterol levels in those with diabetes.27,32–34 A multifactorial approach to diabetic care that includes glucose control; blood pressure management with reninangiotensin system blockers; aspirin therapy; and lipid management with statins has been shown to reduce vascular complications and cardiovascular mortality.32
Antiplatelet Agents
Antiplatelet agents are recommended in all patients for the secondary prevention of CAD. In a large meta-analysis, antiplatelet therapy reduced recurrent vascular events by one fourth in patients with a previous vascular event.35 Aspirin treatment (81 to 162 mg per day) should begin immediately after diagnosis of CAD and continue indefinitely unless contraindicated.2,4,35 Clopidogrel (Plavix) is an effective alternative in patients who cannot take aspirin, and the AHA recommends using clopidogrel in combination with aspirin for up to 12 months after an acute cardiac event or percutaneous coronary intervention (PCI) with stent placement.35,36
Lipid Management
Recent clinical trials have demonstrated that reducing cholesterol levels decreases the risk of recurrent coronary events, and evidence-based cholesterol-lowering guidelines have been established by the National Cholesterol Education Program Adult Treatment Panel III (ATP III).37–39 The AHA and ATP III recommend that all patients with CAD initiate lipid management through therapeutic lifestyle changes.2,4,38 For the secondary prevention of CAD, ATP III recommends LDL levels of less than 100 mg per dL (2.59 mmol per L), with an optional goal of less than 70 mg per dL (1.81 mmol per L); if the LDL level is greater than 130 mg per dL (3.37 mmol per L), cholesterol-lowering medications are indicated in addition to lifestyle changes.38
Statins should be the initial medication choice; however, additional agents may be considered if the LDL goal is not reached through statin therapy alone.2,37,38 Recent studies have shown intensive statin therapy reduces all-cause mortality in patients after acute coronary syndromes compared with standard therapy; consequently, some have encouraged statin use in all patients who have CAD.40,41 For every sustained 2 mg per dL reduction in LDL cholesterol, statin therapy has been shown to reduce major coronary events, coronary revascularization, and stroke by 1 percent.41 The AHA suggests that physicians consider advising patients to increase dietary intake of omega-3 fatty acids to improve cholesterol levels,42 but a Cochrane review found insufficient evidence to recommend for or against supplementation.43
Influenza Vaccination
Influenza vaccination has been shown to reduce the risk of hospitalizations for heart disease and all-cause mortality in older persons, and annual influenza vaccination is recommended by the AHA for patients with CAD.44–46 However, a recent Cochrane review concluded that the data were insufficient to evaluate the effect of vaccination in the secondary prevention of CAD.47
Depression
Observational studies have shown that depression is about three times more common in patients after having an MI than in the general population, and 15 to 20 percent of hospitalized patients with acute MI meet criteria for major depression.48 Studies have shown that depression is associated with a higher risk of recurrent cardiac events one to two years after an MI.48,49 Results of a retrospective review showed that patients with CAD who were depressed and treated with a selective serotonin reuptake inhibitor (SSRI) were 42 percent less likely to experience recurrent MI or death compared with patients who had depression but did not take an antidepressant.48,49 However, a subsequent randomized trial in patients who had an MI found that treatment with an SSRI and cognitive behavior therapy (CBT) did not reduce mortality,50 and the authors of a recent systematic review concluded that treatment of depression with medication or CBT in patients with cardiovascular disease is associated with modest improvement in depressive symptoms, but no improvement in cardiac outcomes.51 The AHA recommends screening for depression during secondary prevention of CAD and, if diagnosed, beginning appropriate treatment.2,4,48,50
PCI and Coronary Artery Bypass Grafting
Interventional treatment options for the secondary prevention of CAD include surgical revascularization by coronary artery bypass grafting (CABG) or PCI. No standardized assessment tool exists, but several factors influence decision making, including the extent of CAD, the severity of ischemia on noninvasive testing, and the presence of left ventricular dysfunction.2 The AHA recommends that persons with CAD undergo risk stratification by exercise stress testing with left ventricular functional assessment or radionuclide myocardial perfusion imaging to identify who would benefit from surgical intervention.23
The role of PCI in the secondary prevention of CAD is limited. Clinical trials involving patients with stable CAD have not shown that PCI prevents further events.36,52,53 One recent trial showed no difference between optimal medical therapy with PCI versus optimal medical therapy alone for death or recurrent cardiovascular events52; however, PCI remains indicated for treatment of angina in select patients because there may be transient improvement in physical limitations, angina frequency, and quality of life.36,52,53 Current guidelines support obtaining objective evidence of ischemia before elective PCI.36,52–54
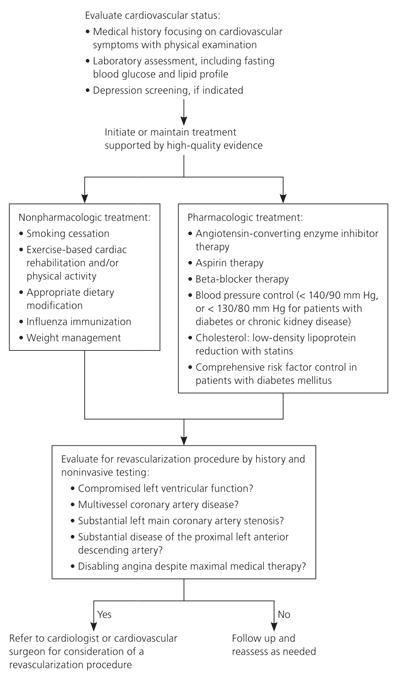
CABG has been shown to reduce mortality in patients who have established CAD with appropriate findings on noninvasive testing (Table 3).23 For those without indications for CABG, medical therapy should be optimized to minimize disease progression.2,23 Despite appropriate medical management, disease progression remains a possibility, and surgical revascularization can be reconsidered based on symptoms and clinical assessment.23 Figure 1 provides an algorithm of evaluation and treatment considerations for the secondary prevention of CAD.2,23
| CABG is recommended for patients with: |
| Disabling angina despite maximal medical therapy, given acceptable surgical risk (if angina is atypical, obtain objective evidence of ischemia) |
| Significant proximal LAD stenosis (≥ 70 percent diameter) |
| Substantial left main coronary artery stenosis |
| 1- or 2-vessel CAD without proximal LAD stenosis, but with a large area of viable myocardium and high-risk criteria on noninvasive testing |
| 2-vessel CAD with significant proximal LAD stenosis and either ejection fraction < 50 percent or ischemia on noninvasive testing |
| 3-vessel CAD (especially if left ventricular ejection fraction < 50 percent) |
| CABG may be considered for patients with: |
| Proximal LAD stenosis with 1-vessel CAD |
| 1- or 2-vessel CAD without significant proximal LAD stenosis, but with moderate area of viable myocardium and demonstrable ischemia on noninvasive testing |
| CABG is not recommended for patients with: |
| Borderline coronary artery stenosis (< 60 percent diameter) in locations other than the left main coronary artery, and no demonstrable ischemia on noninvasive testing |
| Insignificant coronary artery stenosis (< 50 percent diameter) |
| 1- or 2-vessel CAD without significant proximal LAD stenosis, mild symptoms unlikely caused by ischemia, or inadequate trial of medical therapy and a small area of viable myocardium or no demonstrable ischemia on noninvasive testing |