Am Fam Physician. 2010;81(11):1361-1366
Author disclosure: Nothing to disclose.
The increasing use of cross-sectional imaging has led to an increase in the incidental discovery of adrenal masses (adrenal incidentalomas). Although most of these lesions are benign, they often present a diagnostic dilemma. Before creating a management plan, the physician should determine if the lesion is benign or malignant and if the lesion is functioning or nonfunctioning. Incidentally discovered adrenal masses usually are benign adenomas; however, myelolipomas, cysts, hemorrhage, pheochromocytomas, metastases, and adrenocortical carcinomas are also possible. Unenhanced computed tomography and chemical shift magnetic resonance imaging can characterize most adenomas because the lesions have high lipid content. Contrast-enhanced computed tomography can further characterize the adenomas because of the washout characteristics with iodinated intravenous contrast media. Fluorodeoxyglucose–positron emission tomography can be helpful in characterizing some lesions, and biopsy is rarely required. This article summarizes the American College of Radiology Appropriateness Criteria for the use of imaging modalities and biopsy to characterize incidentally discovered adrenal masses.
The increasing use of computed tomography (CT) and magnetic resonance imaging (MRI) has led to a rise in the incidental discovery of adrenal masses (adrenal incidentalomas). Approximately 3 to 4 percent of CT and MRI studies of the abdomen reveal an adrenal incidentaloma.1,2 The most common incidentally discovered adrenal masses are adenomas, which are benign lesions. The prevalence of adenomas rises from 0.2 percent in persons in their twenties to 7 percent in those older than 70 years.3,4 Other adrenal masses that may be encountered incidentally include myelolipomas, cysts, hemorrhage, pheochromocytomas, metastases, and adrenocortical carcinomas.
Evaluation
Cysts, myelolipomas, and adrenal hemorrhage are benign and often can be characterized on the initial imaging study; however, other lesions cannot be easily characterized without the use of more dedicated, specific imaging techniques. A lesion measuring at least 1 cm is normally considered large enough for further imaging investigation.5 There are no data for determining the accuracy of imaging for masses measuring less than 1 cm. Based on anecdotal evidence, most physicians and radiologists believe that masses measuring less than 1 cm do not require further imaging workup. Density measurements with CT may be unreliable at this size. However, patients with small lesions may need hormonal evaluation to determine if they have adrenal hyperfunction.6
The purpose of further imaging and clinical workup is to answer two questions: Is the lesion benign or malignant? Is the lesion functioning or nonfunctioning? Examples of hyperfunctioning lesions include pheochromocytoma, and nodules that secrete cortisol (Cushing syndrome) or aldosterone (Conn syndrome).
Patients with an incidentally discovered adrenal mass are typically asymptomatic, but the mass may later prove to be functional. Although the initial evaluation should always include a thorough history, physical examination, and biochemical evaluation when indicated, this article focuses on radiologic studies based on the American College of Radiology Appropriateness Criteria for initiating further imaging (Table 1).7
| Clinical condition: Incidentally discovered adrenal mass | |||
|---|---|---|---|
| Radiologic procedure | Rating | Comments | RRL |
| Variant 1: No history of malignancy; mass 1–4 cm in diameter. Initial evaluation. | |||
| CT abdomen without contrast | 8 | Presumes that a noncontrast CT has not already been performed or if there are suspicious imaging features | Med |
| CT abdomen without and with contrast | 8 | Indicated if noncontrast CT is not diagnostic AND if there are concerning imaging features of malignancy | Med |
| MRI abdomen without contrast | 8 | May be helpful when nonenhanced CT is equivocal or if there are suspicious imaging features | None |
| Biopsy adrenal gland | 6 | A biopsy should only be performed if the lesion is enlarging and if pheochromocytoma is excluded; CT or US guidance could be used | NS |
| MIBG | 2 | Only for suspicion of pheochromocytoma | High |
| MRI abdomen with contrast | 2 | — | None |
| MRI abdomen without and with contrast | 2 | — | None |
| Iodocholesterol scan | 1 | This agent may be used to detect functionally active adenomas | High |
| FDG-PET whole body | 1 | — | High |
| X-ray abdomen | 1 | — | Med |
| US adrenal gland | 1 | — | None |
| Variant 2: No history of malignancy; mass 1–4 cm in diameter. Follow-up evaluation in 12 months. | |||
| CT abdomen without contrast | 8 | — | Med |
| MRI abdomen without contrast | 8 | — | None |
| CT abdomen without and with contrast | 1 | — | Med |
| MRI abdomen without and with contrast | 1 | — | None |
| Variant 3: No history of malignancy; mass > 4 cm in diameter. (If not typical for adenoma, myelolipoma, hemorrhage, or simple cyst, consider resection.) | |||
| CT abdomen with contrast | 8 | As part of preoperative staging | Med |
| MRI abdomen with contrast | 8 | As part of preoperative staging | None |
| FDG-PET whole body | 5 | As part of preoperative staging | High |
| MIBG | 2 | Only for suspicion of pheochromocytoma | High |
| CT abdomen without and with contrast | 2 | — | Med |
| MRI abdomen without and with contrast | 2 | — | None |
| CT abdomen without contrast | 1 | — | Med |
| MRI abdomen with contrast | 1 | — | None |
| Iodocholesterol scan | 1 | This agent may be used to detect functionally active adenomas | High |
| Biopsy adrenal gland | 1 | — | NS |
| X-ray abdomen | 1 | — | Med |
| US adrenal gland | 1 | — | None |
| Variant 4: History of malignancy; mass < 4 cm in diameter. Initial evaluation. | |||
| CT abdomen without contrast | 8 | If there is no prior imaging and assuming that a noncontrast CT has not already been performed | Med |
| CT abdomen without and with contrast | 8 | Indicated if noncontrast CT is indeterminate (density > 10 HU) or lesion does not lose signal on out-of-phase images | Med |
| MRI abdomen without contrast | 8 | If there is no prior imaging and no prior chemical shift MRI and if washout on dedicated adrenal CT is not diagnostic of adenoma | None |
| Biopsy adrenal gland | 8 | A biopsy should only be performed if imaging characteristics cannot characterize mass as benign and if pheochromocytoma is excluded; CT or US guidance could be used | NS |
| FDG-PET whole body | 8 | If CT and MRI features are not diagnostic of benign lesion and there is no prior imaging | High |
| MIBG | 2 | Only for suspicion of pheochromocytoma | High |
| Iodocholesterol scan | 1 | This agent may be used to detect functionally active adenomas | High |
| X-ray abdomen | 1 | — | Med |
| US adrenal gland | 1 | — | None |
| MRI abdomen with contrast | 1 | — | None |
| MRI abdomen without and with contrast | 1 | — | None |
| Variant 5: History of malignancy; mass > 4 cm in diameter. | |||
| Biopsy adrenal gland | 8 | — | NS |
| FDG-PET whole body | 8 | — | High |
| CT abdomen with contrast | 1 | — | Med |
| MIBG | 1 | — | High |
| MRI abdomen with contrast | 1 | — | None |
| Iodocholesterol scan | 1 | — | High |
| X-ray abdomen | 1 | — | Med |
| US adrenal gland | 1 | — | None |
Is the Mass Benign or Malignant?
The prevalence of primary adrenocortical carcinoma is about 0.06 percent in the general population. A study of 342 patients showed only a 1.2 percent rate of adrenocortical carcinoma in those with adrenal incidentalomas.1 All of the malignant lesions were greater than 5 cm. In general, the larger the mass, the greater the likelihood of malignancy. Approximately 90 percent of adrenocortical carcinomas are larger than 4 cm when first discovered.8
In the absence of a history of malignancy, the discovery of a metastatic lesion in the adrenal gland is rare, occurring in 0.3 percent of patients with incidentalomas.1,9 In persons with known malignancy, the incidence is higher at 25 to 36 percent.10–12 Bronchogenic carcinoma, renal cell carcinoma, and melanoma represent the most common primary malignancies with adrenal metastases.
A CT or MRI can usually determine whether an adrenal incidentaloma is benign by detecting lipid in the mass (Table 2). Adenomas usually contain lipid (lipid-rich adenomas); however, most lipid-poor lesions are also adenomas.1,13 Only a small proportion of lipid-poor lesions are malignant nonadenomas. Unenhanced CT cannot reliably distinguish between lipid-poor adenomas and nonadenomas; however, contrast-enhanced CT with washout calculations has been shown to help make this distinction with high specificity.14–17 Adenomas (both lipid-rich and lipid-poor) demonstrate rapid washout of the intravenously injected, iodinated contrast media, as opposed to malignant masses.14–17
| Type of incidentaloma* | Findings | ||||
|---|---|---|---|---|---|
| Size | Attenuation on unenhanced CT | Rapidity of washout of contrast media on enhanced CT | Appearance on MRI (out-of-phase image) | Growth rate | |
| Lipid-rich adrenal adenoma | < 3 cm | ≤ 10 HU | Rapid washout | Signal loss | Size usually stable |
| Lipid-poor adrenal adenoma | < 3 cm | > 10 HU | Rapid washout | No signal loss | Size usually stable |
| Adrenocortical carcinoma | Usually > 5 cm | > 10 HU | No rapid washout | No signal loss | Usually significant growth |
| Pheochromocytoma | Variable size | > 10 HU | No rapid washout | No signal loss | Slow growth |
| Metastasis | Variable size | > 10 HU | No rapid washout | No signal loss | Usually significant growth |
Imaging to Determine Malignancy
Most incidentally detected adrenal masses greater than 1 cm are characterized as benign, lipid-rich adenomas using the unenhanced phase of adrenal protocol CT (Figure 1). For masses that do not fit into this category, further contrast-enhanced and delayed-phase CT with washout calculations can be performed as part of the same examination. For patients who are unable to undergo a contrast-enhanced CT because of renal insufficiency or allergy to iodinated contrast media, chemical shift MRI can be performed. CT and MRI demonstrate similar accuracy in the diagnosis of adrenal masses.18,19 Cost and availability determine which is used as the primary modality in individual centers.
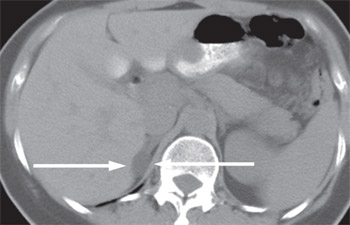
Fluorodeoxyglucose–positron emission tomography (FDG-PET) can be used for lesions that cannot be categorized by CT or MRI. If the lesion still cannot be characterized, biopsy or surgical removal may be indicated.
The tracer m-iodobenzylguanidine (MIBG) localizes to adrenergic nerve endings, and studies using MIBG are useful in patients with suspected pheochromocytoma.
CT
The Hounsfield unit scale is a semiquantitative method of measuring radiography attenuation. Organs and tissues demonstrate variable attenuation depending on their density. Fat- and lipid-containing lesions have lower densities than the spleen, normal adrenal gland, or parenchyma of the liver. If an adrenal mass measures 10 HU or less on unenhanced CT, it is probably a lipid-rich adenoma.14 The mean Hounsfield unit for adrenal carcinoma, metastasis, and pheochromocytoma is significantly higher.20 A threshold value of 10 HU has a 71 percent sensitivity and 98 percent specificity for identifying adenomas and is generally accepted as a cutoff for diagnosis of lipid-rich adenomas.21,22
MRI
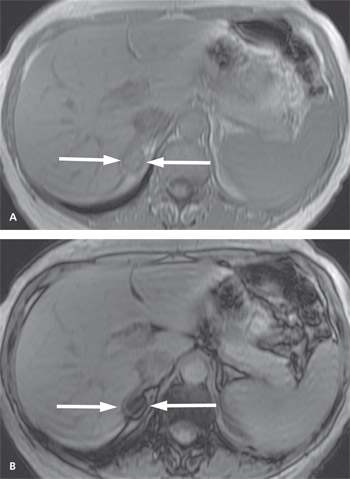
Chemical shift MRI uses a technique based on hydrogen and fat protons, which resonate at different frequencies. By using different time parameters during the same MRI examination, it is possible to identify lipid-rich adenomas. These adenomas show signal loss on out-of-phase imaging, as opposed to imaging when the protons are in phase (Figure 2). In contrast, nonadenomas do not show signal loss on out-of-phase imaging.23,24 Recent studies have shown that 60 to 89 percent of lesions measuring between 10 and 30 HU on unenhanced CT can be characterized using chemical shift MRI.23,25
FDG-PET
Evaluation using FDG-PET has a high sensitivity for detecting malignancy 26; however, adenomas can also occasionally take up the radiotracer, decreasing the test's specificity. Therefore, FDG-PET is used to determine the need for biopsy in masses that cannot be characterized on CT or MRI.
ADRENAL BIOPSY TO DETECT MALIGNANCY
The role of adrenal biopsy has evolved. Biopsy is not performed to diagnose adrenal adenoma, but mostly to confirm or rule out malignancy in the few cases in which CT or MRI results are equivocal. However, before adrenal biopsy is performed, pheochromocytoma should be excluded with biochemical tests, especially in an incidentally detected mass, because hemorrhage and hypertensive crisis have been reported as complications of pheochromocytomas biopsy.27,28
Is the Lesion Functioning or Nonfunctioning?
Subclinical hyperfunctioning adrenal nodules are well recognized; pheochromocytomas, aldosteronomas, and cortisol-secreting nodules are the most common.13,29 Although the literature yields variable results, the incidence of subclinical hyperfunctioning adrenal lesions is between 0.4 and 15 percent. In one study, 19 of 33 adrenal pheochromocytomas were detected incidentally, and only 10 of the 19 patients were hypertensive.30 Although a functioning mass is often suggested by the history and physical examination findings, the absence of these findings does not exclude a hormonally active lesion. Studies have shown detectable secretion of aldosterone, cortisol, or catecholamines in 5 to 23 percent of patients with adrenal incidentalomas.31–34 Table 3 outlines the biochemical workup to help determine if the lesion is functioning.5
| Possible cause | Incidentalomas with this diagnosis (%) | Clinical signs | Laboratory tests |
|---|---|---|---|
| Cushing syndrome | 5 | Hypertension, moon-shaped face, striae, proximal muscle weakness, truncal obesity, thin skin, easy bruising | Overnight dexamethasone suppression test, serum and 24-hour urinary cortisol levels, serum corticotropin level |
| Pheochromocytoma | 3 to 10 | Hypertension, headache, diaphoresis, palpitations | 24-hour urinary fractionated metanephrine and catecholamines levels |
| Primary aldosteronism | Less than 1 | Hypertension, hypokalemia, hypernatremia | Morning plasma aldosterone concentration and renin activity, aldosterone suppression test |
Follow-up and Treatment
Patients with incidentally discovered adrenal masses measuring greater than 4 cm who do not have a history of malignancy usually undergo surgery if the lesions cannot be characterized as cysts or myelolipomas, because of the higher risk of adrenocortical carcinoma in masses of this size.6 For lesions measuring 1 to 4 cm that are characterized as adenomas, follow-up unenhanced CT at 12 months can be performed to ensure that there has not been significant growth. Tumors that have grown should be further investigated, and adrenal biopsy or resection should be considered, although even growing adrenal lesions are usually not malignant.13 If the size of the mass is stable, no further imaging is required, although serial biochemical evaluation may be needed.35
The National Institutes of Health and the American Association of Clinical Endocrinologists recommend that if autonomous function is not present on the initial study, hormonal evaluation should be repeated annually for at least four years.5,6,13,35,36 Benign lesions that demonstrate autonomous function are managed either medically or surgically depending on the clinical circumstances.37,38 Adrenal cysts and myelolipomas usually do not need follow-up; however, surgical evaluation may be needed if the mass is large enough to cause symptoms.