
Am Fam Physician. 2010;82(2):178-179
A more recent USPSTF on depression in children and adolescents is available.
Summary of Recommendations and Evidence
The U.S. Preventive Services Task Force (USPSTF) recommends screening adolescents (12 to 18 years of age) for major depressive disorder (MDD) when systems are in place to ensure accurate diagnosis, psychotherapy (i.e., cognitive behavioral or interpersonal), and follow-up (Table 1).B recommendation.
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening children (seven to 11 years of age) for MDD. I statement.
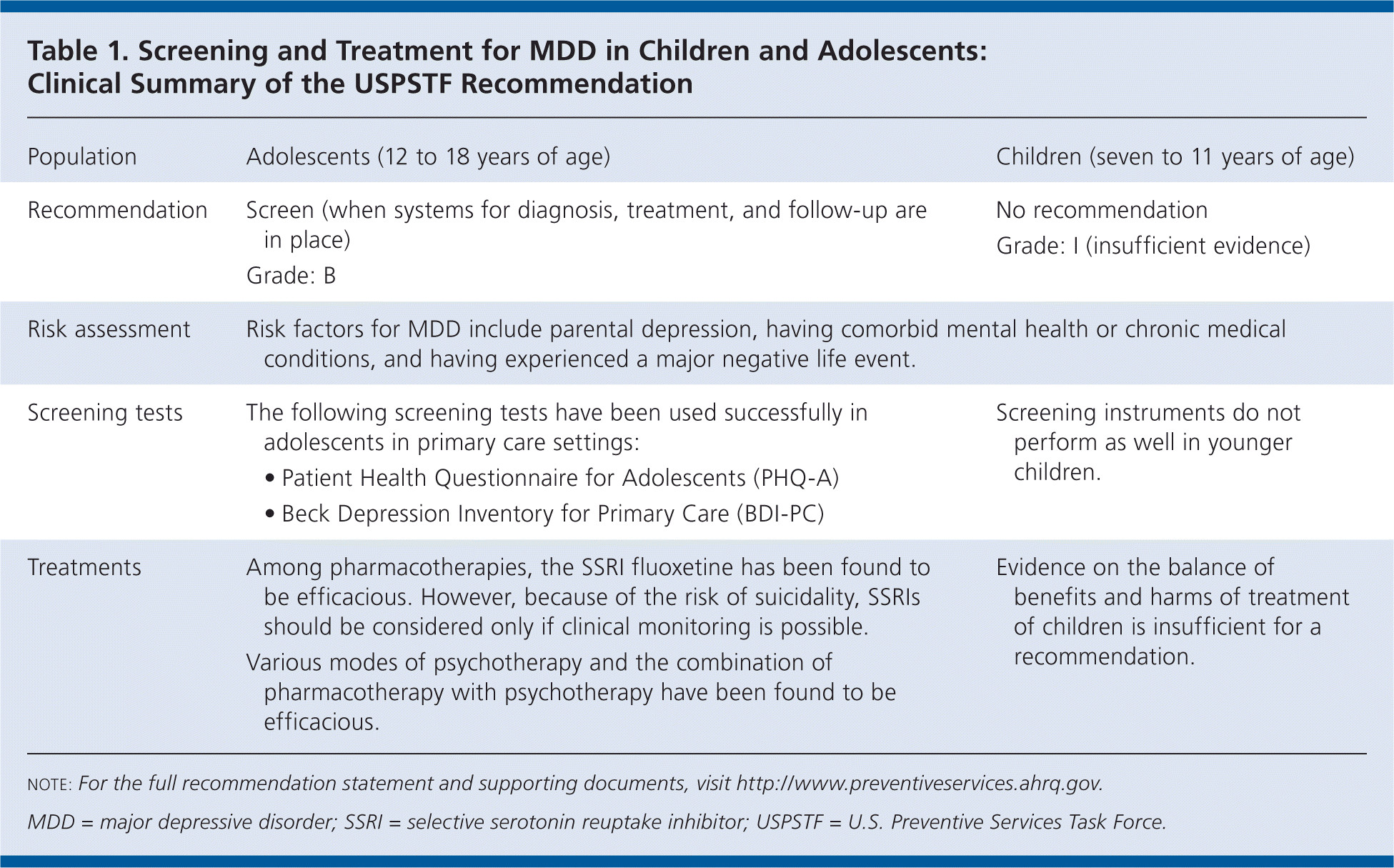
| Population | Adolescents (12 to 18 years of age) | Children (seven to 11 years of age) |
| Recommendation | Screen (when systems for diagnosis, treatment, and follow-up are in place) Grade: B | No recommendation Grade: I (insufficient evidence) |
| Risk assessment | Risk factors for MDD include parental depression, having comorbid mental health or chronic medical conditions, and having experienced a major negative life event. | |
| Screening tests | The following screening tests have been used successfully in adolescents in primary care settings:
| Screening instruments do not perform as well in younger children. |
| Treatments | Among pharmacotherapies, the SSRI fluoxetine has been found to be efficacious. However, because of the risk of suicidality, SSRIs should be considered only if clinical monitoring is possible. Various modes of psychotherapy and the combination of pharmacotherapy with psychotherapy have been found to be efficacious. | Evidence on the balance of benefits and harms of treatment of children is insufficient for a recommendation. |
Rationale
Importance. MDD among youth is a disabling condition that is associated with serious long-term morbidities and risk of suicide. However, the majority of youth with depression are undiagnosed and untreated.
Detection. There is adequate evidence that screening tests accurately identify MDD in adolescents. The USPSTF found inadequate evidence that screening tests accurately identify MDD in children.
Benefits of detection and early intervention. Adolescents (12 to 18 years of age): The USPSTF found adequate evidence that treatment with selective serotonin reuptake inhibitors (SSRIs), psychotherapy, or combined therapy (SSRIs and psychotherapy) leads to decreases in MDD symptoms in adolescents.
Children (seven to 11 years of age): The USPSTF found inadequate evidence to support the benefits of treating MDD in children. SSRIs (fluoxetine) reduce MDD symptoms in children; however, there are limited data on the benefits of psychotherapy and the benefits of psychotherapy plus SSRIs.
Harms of detection and early treatment. Adolescents: There is convincing evidence that taking SSRIs may lead to harms (risk of suicidality [i.e., suicide ideation, preparatory acts, or suicide attempts]) in adolescents. Limited evidence exists regarding the harms of combining SSRIs and psychotherapy. However, there is inadequate evidence about the harms of screening and psychotherapy in adolescents, which are probably small.
Children: SSRIs (fluoxetine) demonstrated harms in children (risk of suicidality); however, there is limited evidence on the harms of psychotherapy or the harms of combining of psychotherapy and SSRIs (fluoxetine). There is also limited evidence about the harms of screening children. The USPSTF judged that the overall evidence is inadequate regarding the harms of screening and treatment of MDD in children.
USPSTF assessment. The USPSTF concludes that:
In adolescents (12 to 18 years of age), there is moderate certainty that the net benefit of psychotherapy is moderate.
In children (seven to 11 years of age), the evidence is lacking, and the balance of benefits and harms of psychotherapy cannot be determined.
Clinical Considerations
Patient population. This USPSTF recommendation addresses screening for MDD in adolescents and children in the general population. There is a spectrum of depressive disorders. This report focuses only on screening for MDD and does not address screening for various less severe depressive disorders.
Assessment of risk. A variety of factors contribute to the development of MDD. Most persons who develop MDD have multiple risk factors. However, risk factors for MDD can be difficult to assess. As a result, researchers have focused on identifying youth subgroups at increased risk of developing MDD. Important risk factors that can be assessed relatively accurately and reliably include parental depression, having comorbid mental health or chronic medical conditions, and having experienced a major negative life event.
Screening tests. Instruments developed for primary care (e.g., Patient Health Questionnaire for Adolescents [PHQ-A], the Beck Depression Inventory for Primary Care [BDI-PC]) have been used successfully in adolescents. There are limited data describing the accuracy of using MDD screening instruments in children.
Treatment. Among pharmacothera-pies available for the treatment of MDD in children and adolescents, SSRIs have been found to be efficacious. Treating youth with depression using SSRIs is associated with an increased risk of suicidality, and therefore should be considered only if judicious clinical monitoring is possible. Psychotherapy trials indicate that a variety of psychotherapy types are efficacious among adolescents, including cognitive behavioral and interpersonal therapies. Harms of psychotherapy are believed to be small.
