
Am Fam Physician. 2010;82(4):381-388
Patient information: See related handout on the side effects of radiation therapy, written by the author of this article.
Author disclosure: Nothing to disclose.
Nearly two thirds of patients with cancer will undergo radiation therapy as part of their treatment plan. Given the increased use of radiation therapy and the growing number of cancer survivors, family physicians will increasingly care for patients experiencing adverse effects of radiation. Selective serotonin reuptake inhibitors have been shown to significantly improve symptoms of depression in patients undergoing chemotherapy, although they have little effect on cancer-related fatigue. Radiation dermatitis is treated with topical steroids and emollient creams. Skin washing with a mild, unscented soap is acceptable. Cardiovascular disease is a well-established adverse effect in patients receiving radiation therapy, although there are no consensus recommendations for cardiovascular screening in this population. Radiation pneumonitis is treated with oral prednisone and pentoxifylline. Radiation esophagitis is treated with dietary modification, proton pump inhibitors, promotility agents, and viscous lidocaine. Radiation-induced emesis is ameliorated with receptor antagonists and steroids. Symptomatic treatments for chronic 5-hydroxytryptamine3 radiation cystitis include anticholinergic agents and phenazopyridine. Sexual dysfunction from radiation therapy includes erectile dysfunction and vaginal stenosis, which are treated with phosphodiesterase type 5 inhibitors and vaginal dilators, respectively.
Physicians diagnosed an estimated 1,480,000 new cancer cases in the United States in 2009 (excluding basal cell and squamous cell skin cancers, and carcinomas in situ, except urinary bladder).1 Nearly two thirds of these patients have treatment plans that may involve radiation therapy.2 Radiation therapy is used in curative, palliative, and prophylactic treatment plans, and is delivered through external beam, internal placement, or systemic administration, depending on the type of cancer and treatment goals. Radiation therapy works by damaging a cell's DNA and inhibiting its ability to reproduce. Healthy, noncancerous cells usually recover after radiation therapy, whereas cancerous cells are often unable to repair the radiation-induced damage. Careful treatment planning, including the use of radiosensitizers and radioprotectants, aims to limit radiation exposure to noncancerous cells, thus limiting adverse effects. Table 1 lists adverse effects of radiation therapy, including risk factors and treatment recommendations.3–48
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Selective serotonin reuptake inhibitors improve depression in patients receiving chemotherapy. | B | 6, 7 | RCTs of paroxetine (Paxil) |
| Patients with cancer should be assessed for fatigue at regular intervals. | C | 51 | Recommendation from consensus guideline |
| 5-hydroxytryptamine3 receptor antagonists (such as ondansetron [Zofran]) are recommended first-line therapy for radiation-induced emesis. | B | 33 | Recommendation from evidence-based practice guideline |
| Adding dexamethasone (4 mg daily for five days) to ondansetron therapy provides additional prophylaxis against radiation-induced emesis. | B | 33, 34 | Consistent findings from small RCT34 and recommendation from evidence-based practice guideline33 |
| Phosphodiesterase type 5 inhibitors, such as sildenafil (Viagra) and tadalafil (Cialis), are effective for erectile dysfunction associated with radiation therapy. | A | 42–44 | Consistent findings from RCTs of sildenafil and tadalafil |
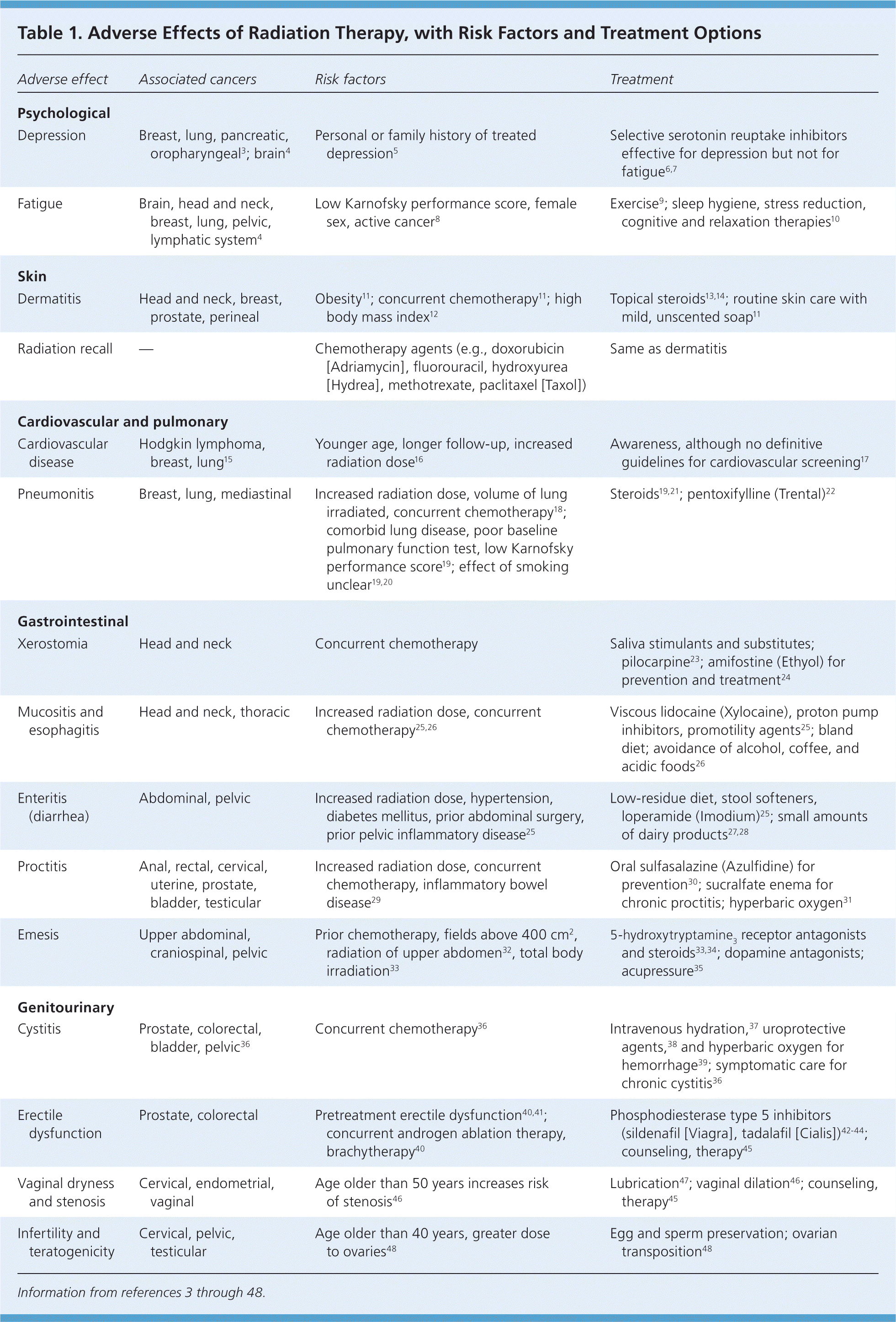
| Adverse effect | Associated cancers | Risk factors | Treatment |
|---|---|---|---|
| Psychological | |||
| Depression | Breast, lung, pancreatic, oropharyngeal3; brain4 | Personal or family history of treated depression5 | Selective serotonin reuptake inhibitors effective for depression but not for fatigue6,7 |
| Fatigue | Brain, head and neck, breast, lung, pelvic, lymphatic system4 | Low Karnofsky performance score, female sex, active cancer8 | Exercise9; sleep hygiene, stress reduction, cognitive and relaxation therapies10 |
| Skin | |||
| Dermatitis | Head and neck, breast, prostate, perineal | Obesity11; concurrent chemotherapy11; high body mass index12 | Topical steroids13,14; routine skin care with mild, unscented soap11 |
| Radiation recall | — | Chemotherapy agents (e.g., doxorubicin [Adriamycin], fluorouracil, hydroxyurea [Hydrea], methotrexate, paclitaxel [Taxol]) | Same as dermatitis |
| Cardiovascular and pulmonary | |||
| Cardiovascular disease | Hodgkin lymphoma, breast, lung15 | Younger age, longer follow-up, increased radiation dose16 | Awareness, although no definitive guidelines for cardiovascular screening17 |
| Pneumonitis | Breast, lung, mediastinal | Increased radiation dose, volume of lung irradiated, concurrent chemotherapy18; comorbid lung disease, poor baseline pulmonary function test, low Karnofsky performance score19; effect of smoking unclear19,20 | Steroids19,21; pentoxifylline (Trental)22 |
| Gastrointestinal | |||
| Xerostomia | Head and neck | Concurrent chemotherapy | Saliva stimulants and substitutes; pilocarpine23; amifostine (Ethyol) for prevention and treatment24 |
| Mucositis and esophagitis | Head and neck, thoracic | Increased radiation dose, concurrent chemotherapy25,26 | Viscous lidocaine (Xylocaine), proton pump inhibitors, promotility agents25; bland diet; avoidance of alcohol, coffee, and acidic foods26 |
| Enteritis (diarrhea) | Abdominal, pelvic | Increased radiation dose, hypertension, diabetes mellitus, prior abdominal surgery, prior pelvic inflammatory disease25 | Low-residue diet, stool softeners, loperamide (Imodium)25; small amounts of dairy products27,28 |
| Proctitis | Anal, rectal, cervical, uterine, prostate, bladder, testicular | Increased radiation dose, concurrent chemotherapy, inflammatory bowel disease29 | Oral sulfasalazine (Azulfidine) for prevention30; sucralfate enema for chronic proctitis; hyperbaric oxygen31 |
| Emesis | Upper abdominal, craniospinal, pelvic | Prior chemotherapy, fields above 400 cm2, radiation of upper abdomen32, total body irradiation33 | receptor antagonists 5-hydroxytryptamine3 and steroids33,34; dopamine antagonists; acupressure35 |
| Genitourinary | |||
| Cystitis | Prostate, colorectal, bladder, pelvic36 | Concurrent chemotherapy36 | Intravenous hydration,37 uroprotective agents,38 and hyperbaric oxygen for hemorrhage39; symptomatic care for chronic cystitis36 |
| Erectile dysfunction | Prostate, colorectal | Pretreatment erectile dysfunction40,41; concurrent androgen ablation therapy, brachytherapy40 | Phosphodiesterase type 5 inhibitors (sildenafil [Viagra], tadalafil [Cialis])42–44; counseling, therapy45 |
| Vaginal dryness and stenosis | Cervical, endometrial, vaginal | Age older than 50 years increases risk of stenosis46 | Lubrication47; vaginal dilation46; counseling, therapy45 |
| Infertility and teratogenicity | Cervical, pelvic, testicular | Age older than 40 years, greater dose to ovaries48 | Egg and sperm preservation; ovarian transposition48 |
For most types of cancer, at least 75 percent of patients receiving radiation therapy are treated with intent to cure.2 Thus, with increasing numbers of cancer survivors and a relative lack of subspecialists, family physicians play an important role in cancer follow-up care.49 Although early radiation toxicities are usually part of the subspecialist's care, patients with late effects often present to the family physician.
Adverse effects from radiation therapy are classified as early or late. Early adverse effects occur during treatment or just after its completion, and usually resolve within four to six weeks. Late adverse effects are noted months to years after treatment completion and are often permanent. Secondary malignancies from radiation therapy, usually manifesting 10 to 15 years after treatment, are proportional to the amount of radiation received and inversely proportional to the age at which the radiation was received.50
Depression and Fatigue
The prevalence of depression among patients with cancer varies from zero to 60 percent, depending on criteria, methodology, and populations studied. Depression is highly associated with brain, breast, lung, pancreatic, and oropharyngeal malignancies, and is less associated with colon and gynecologic tumors.3,4 Risk factors include a personal or family history of treated depression.5 Because of comorbidities and combined treatment regimens with other modalities (e.g., chemotherapy), it is difficult to assess the direct effect of radiation on depression. Although selective serotonin reuptake inhibitors (based on studies of paroxetine [Paxil]) appear to significantly improve depressive symptoms among patients undergoing chemotherapy, they have no effect on cancer-related fatigue.6,7
Patients with cancer often find fatigue to be the most distressing adverse effect of radiation therapy, more than the pain, nausea, and vomiting associated with the cancer and treatments.51 All patients with cancer should be assessed for fatigue at regular intervals,51 because radiation-related fatigue occurs in 80 percent of patients acutely and 30 percent chronically.4 Radiation-related fatigue is closely associated with irradiated tumors of the brain, head and neck, breast, lung, pelvis, and lymphatic system.4 Risk factors include low Karnofsky performance score, female sex, and active cancer.8 Exercise is often recommended for those undergoing treatment, and a mild to moderate improvement in fatigue has been demonstrated in some, but not all studies. A small randomized study of patients undergoing radiation therapy for prostate cancer demonstrated less fatigue and improved cardiovascular function in those who completed an eight-week exercise program.9 Proper sleep hygiene, stress reduction techniques, and cognitive and relaxation therapies may be beneficial.10 The use of psychostimulants, including methylphenidate (Ritalin) and modafinil (Provigil), remains investigational, and optimal dosing in patients with cancer has not been established.51 Yoga has been demonstrated to reduce depression, perceived stress, and morning cortisol levels in patients with breast cancer undergoing radiation therapy.52 Additional nonpharmacologic recommendations for managing cancer-related fatigue are listed in Table 2.51

| Activity enhancement* |
| Maintain optimal level of activity |
| Consider exercise program |
| Consider physical or occupational therapy |
| CBT for sleep |
| Sleep restriction (limit naps, total time in bed) |
| Sleep hygiene (avoid caffeine after noon, establish a sleep-conducive environment) |
| Stimulus control (going to bed at same time each night) |
| Energy conservation |
| Set priorities |
| Pace activities |
| Delegate responsibilities |
| Schedule activities at time of peak energy |
| Postpone nonessential activities |
| Limit naps to 20 to 30 minutes or less to minimize interference with nighttime sleep quality |
| Maintain structured daily routine |
| Attend to one activity at a time |
| Psychosocial interventions |
| CBT |
| Stress management |
| Relaxation techniques |
| Support groups |
Radiation Dermatitis
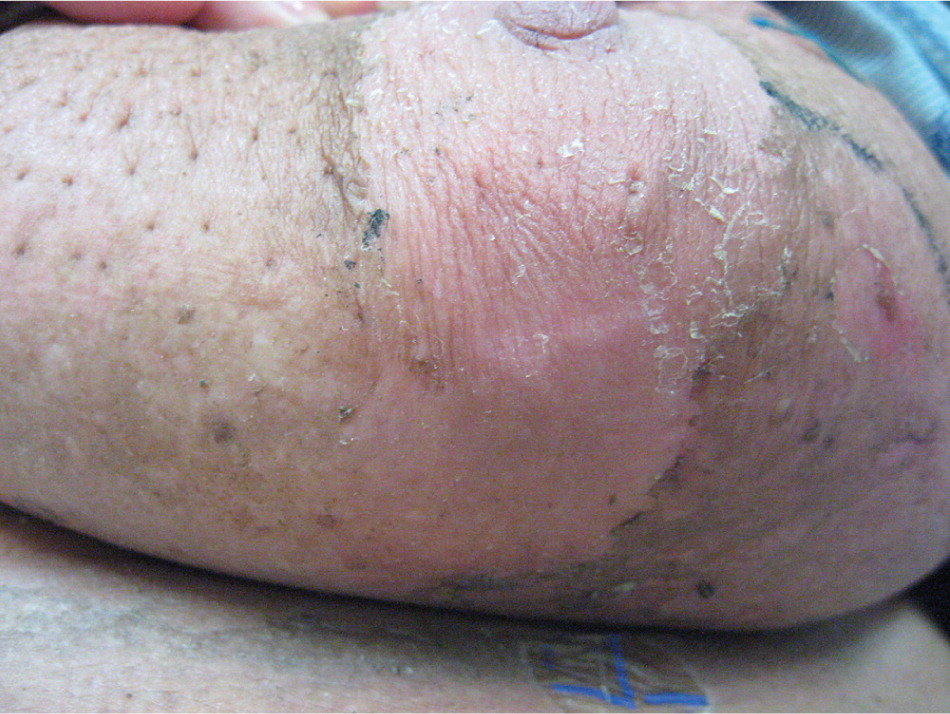
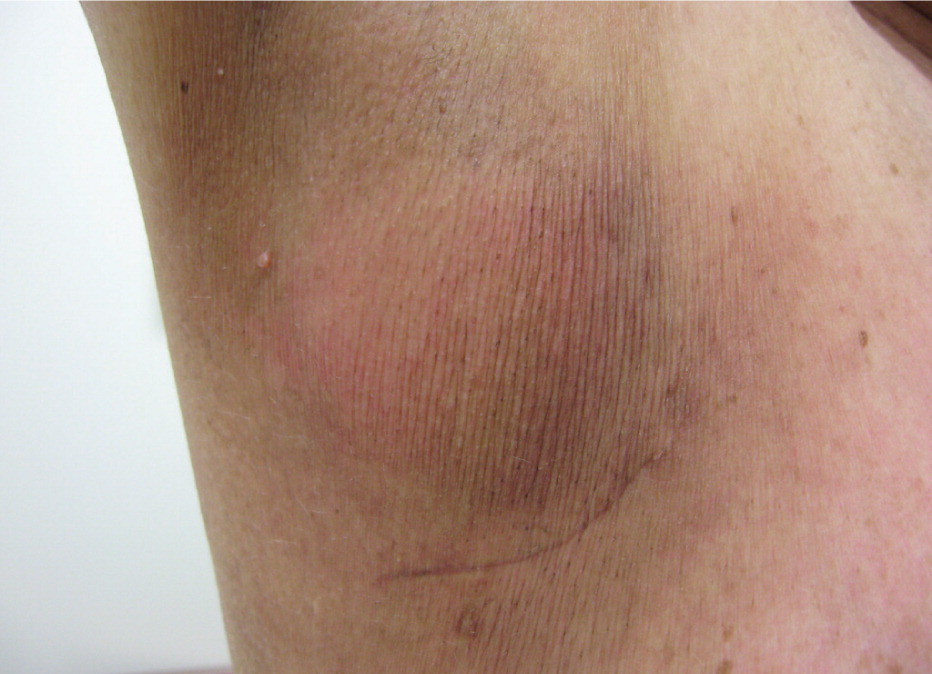
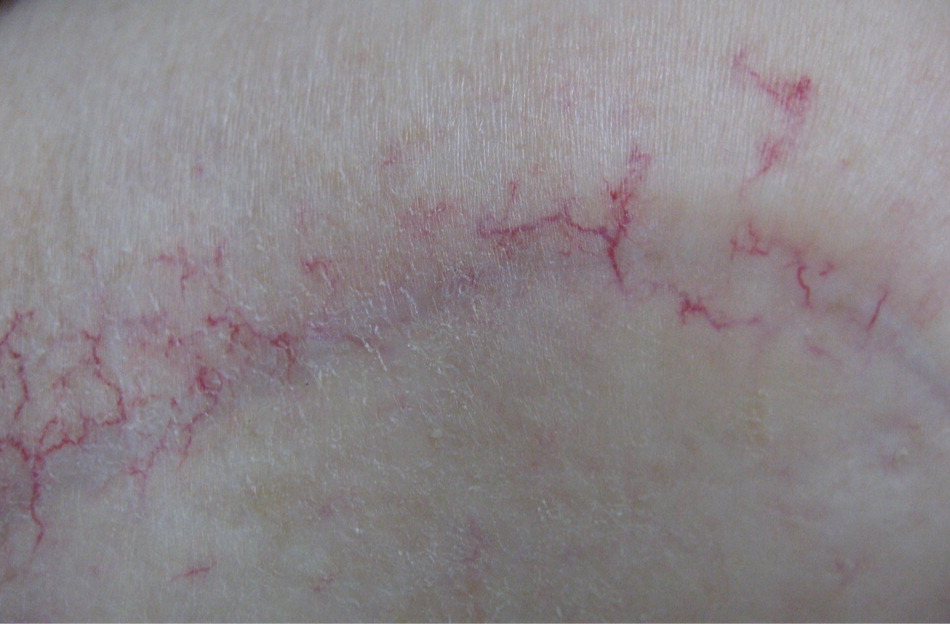
Radiation dermatitis is a common adverse effect of radiation therapy, often complicating treatment of breast, prostate, perineal, and head and neck malignancies. However, there is no general agreement, and there are few controlled trials regarding prevention and treatment.53 Furthermore, clinical practice is often based on anecdotal evidence, and patient advice is variable and often contradictory.54 Early skin changes include erythema (Figure 1), dry desquamation (Figures 2 and 3), and moist desquamation; late adverse effects include pigmentation changes, telangiectasias (Figure 4), hair loss, atrophy, fibrosis, and ulceration (Table 3).55 Risk factors for dermatitis include obesity, concurrent chemotherapy, and high body mass index.11,12 Radiation recall is a phenomenon of rapid onset skin irritation in a previously radiated field shortly after starting certain chemotherapy agents, most notably doxorubicin (Adriamycin), fluorouracil, hydroxyurea (Hydrea), methotrexate, and paclitaxel (Taxol).
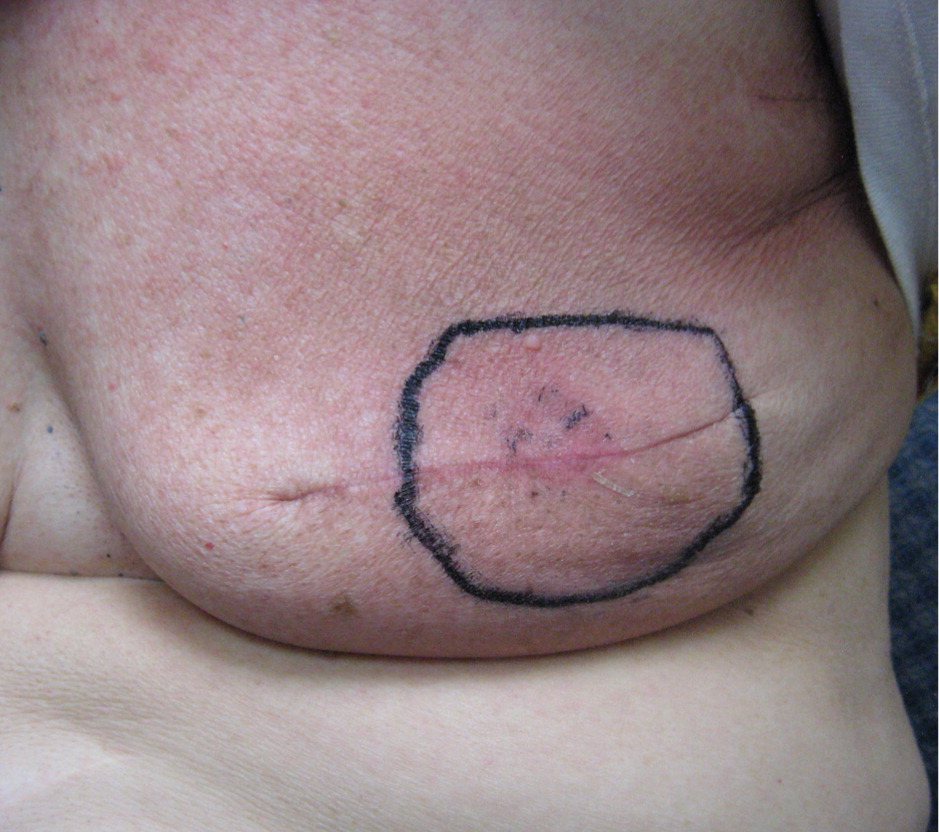

| Early changes | |
| Erythema | |
| Pink coloration | |
| Mild edema | |
| Itching, burning, mild discomfort | |
| Dry desquamation | |
| Partial loss of epidermal basal cells | |
| Dryness, scaling, peeling | |
| Hyperpigmentation | |
| Moist desquamation | |
| Complete destruction of basal cell layer | |
| Blister formation | |
| Nerve pain | |
| Serous drainage | |
| Late changes | |
| Pigmentation changes | |
| Hair loss (permanent) | |
| Telangiectasia | |
| Atrophy | |
| Ulceration | |
| Fibrous changes | |
| Recall phenomenon | |
| Rapid onset and progression after administration of chemotherapy drugs in previously irradiated area | |
| Symptoms of moist desquamation | |
Topical steroids and dexpanthenol-containing emollients are often prescribed for radiation dermatitis, although there is insufficient evidence to firmly support or refute this recommendation.13 A randomized, double-blind study evaluating 0.1% methylprednisolone and 0.5% dexpanthenol demonstrated a mild clinical benefit with both agents when used prophylactically and during treatment, compared with use of no topical creams, although data suggest a slightly greater benefit from the steroid cream.14 Although previous recommendations warned against the use of soap and water on skin in the radiation field, current advice permits use of routine skin washing with a mild, unscented soap.11 Deodorant use does not increase radiodermatitis,56 although antiperspirant with aluminum has not been well-studied. Topical aloe vera gel does not prevent or treat radiation dermatitis.57 Patients undergoing radiation therapy should avoid swimming, especially in chlorinated pools and hot tubs, and use care when applying adhesive bandages on skin in the radiated field.55 Soft tissue radionecrosis and osteoradionecrosis are ameliorated with traditional wound care techniques and, in some cases, hyperbaric oxygen therapy.58
Cardiovascular and Pulmonary Adverse Effects
Cardiovascular disease secondary to radiation therapy is a well-recognized adverse effect, primarily seen in patients who receive radiation for Hodgkin lymphoma and, to a lesser extent, breast and lung cancers.15 The estimated relative risk of fatal cardiovascular events after mediastinal radiation ranges from 2.2 to 7.2 for Hodgkin lymphoma and 1.0 to 2.2 for left-sided breast cancer.16 The risk increases with a younger age at treatment, longer follow-up, and increased radiation dose.16 There are currently no definitive guidelines for cardiovascular screening among such cancer survivors. Additionally, most of the research on secondary malignancies and cardiovascular adverse effects is based on patients who completed now outdated treatments for Hodgkin lymphoma.17 Pericarditis, an uncommon acute adverse effect, is seen primarily in patients treated for Hodgkin lymphoma.16
Radiation pneumonitis is seen in 5 to 15 percent of patients irradiated for breast, lung, and mediastinal tumors.18 The risk of developing radiation pneumonitis is directly related to the volume of irradiated lung, the amount of radiation given, and the use of concurrent chemotherapy.18 Additional risk factors include comorbid lung disease, poor baseline pulmonary function testing, and low Karnofsky performance score.19 Opinions vary regarding the effect of smoking. Some studies identify smoking as a risk for pneumonitis,19 whereas others suggest smoking may lessen the risk.20
Symptoms of radiation pneumonitis, including low-grade fever, congestion, dry cough, pleuritic chest pain, and a sensation of chest fullness, usually develop one to three months after completion of radiation therapy. Diagnosis is difficult, often complicated by comorbid conditions and radiation injury to adjacent structures (e.g., esophagus, pericardium). Prednisone, in dosages of at least 50 to 60 mg per day for one week followed by an extended taper, has been shown to abate symptoms and improve lung function.19,21 Pentoxifylline (Trental) is beneficial in preventing early and late lung toxicity.22 Supplemental oxygen may be necessary. Late pulmonary adverse effects include pulmonary fibrosis, which is often permanent and marked by progressive dyspnea.
Gastrointestinal Toxicity
Xerostomia, resulting from radiation injury of the salivary gland, is common with irradiation of the head and neck, especially with concurrent chemotherapy. Xerostomia is treated conservatively with saliva substitutes (e.g., water or glycerin-based) and saliva stimulants (e.g., sour sweets, chewing gum). Pilocarpine is more effective than artificial saliva, although its effectiveness may not be seen until 12 weeks of therapy.23 Mucositis from radiation damage of the oral epithelium responds to topical anesthetics. Oral candidiasis is treated with topical anti-fungal washes or systemic antifungal agents. Amifostine (Ethyol), a free-radical scavenger radioprotectant, is beneficial in preventing and treating xerostomia, but there is insufficient evidence to support its use for radiation-induced mucositis and esophagitis.24
Radiation esophagitis is a common, dose-limiting, early adverse effect of radiation treatment of thoracic tumors. Its incidence is greater with higher radiation dose and concurrent chemotherapy.25,26 Early symptoms, usually following two to three weeks of treatment, include dysphagia and odynophagia. Topical anesthetics such as viscous lidocaine (Xylocaine), proton pump inhibitors, and promotility agents may provide symptomatic relief.25 Dietary modifications shown to reduce incidence and severity include a bland diet and avoidance of alcohol, coffee, and acidic foods.26
Acute enteritis secondary to radiation therapy is usually a self-limiting process treated conservatively with dietary changes and antidiarrheal medications. Chronic symptoms begin three months or more after completion of radiation therapy and may last indefinitely. Risk of enteritis increases with greater radiation dose, as well as a history of abdominal surgery, pelvic inflammatory disease, hypertension, and diabetes mellitus.25 Mild intermittent symptoms of chronic enteritis are managed with a low-residue diet, stool softeners, and loperamide (Imodium).25 Normal portions (one half cup or larger) of foods with moderate-to-high fiber content can exacerbate symptoms of chronic radiation enteritis.27 Lactose intolerance is common following radiation,28 although patients may tolerate small portions of dairy products.27
Radiation proctitis is seen in radiation treatment for anal, rectal, cervical, uterine, bladder, testicular, and, particularly, prostate cancers. Increased radiation dose and concurrent chemotherapy are risk factors, as is the presence of inflammatory bowel disease in patients receiving external beam radiation to the pelvis.29 Oral sulfasalazine (Azulfidine) is effective in the prevention of proctitis in all patients receiving pelvic radiation.30 Sucralfate enemas are recommended for treatment of chronic radiation-induced proctitis. Hyperbaric oxygen therapy significantly improved healing responses in patients with refractory radiation proctitis in a study of 120 patients (number needed to treat = 3).31
Radiation-Induced Emesis
Risk factors for radiation-induced emesis include previous chemotherapy, radiation of the upper abdomen, and radiation fields greater than 400 cm2.32 Patients at greatest risk are those receiving total-body irradiation. Those at moderate risk include patients with radiation of the upper abdominal, pelvic, and craniospinal fields. Current clinical guidelines recommend the use of 5-hydroxytryptamine3 (5-HT3) receptor antagonists, which have antiemetic properties equal or superior to dopamine antagonists but with a more favorable adverse effect profile.33 The addition of dexamethasone (4 mg daily for five days) to the receptor antagonist 5-HT3ondansetron (Zofran) provides additional benefit for prophylaxis against radiation-induced emesis.34 Loraz-epam (Ativan) and diphenhydramine (Benadryl) are also useful adjuncts, but are not recommended as single agents. Nonpharmacologic strategies, including dietary alteration, hypnosis, and acupressure,35 may benefit patients with radiation-induced emesis.
Radiation Cystitis
Acute radiation cystitis, including the more severe hemorrhagic cystitis, is an uncommon adverse effect of radiation therapy. Concurrent chemotherapy increases the risk.36 Intravenous hydration37 and uroprotective agents, including mesna38 (Mesnex) and amifostine, have demonstrated effectiveness in treating hemorrhagic cystitis, although the studies are primarily in patients receiving chemotherapy. Hyperbaric oxygen therapy has also demonstrated improved outcomes in patients with radiation-induced hemorrhagic cystitis.39 Chronic radiation cystitis occurs most commonly following radiation for prostate, colorectal, bladder, and pelvic tumors. Medical therapy is aimed primarily at symptom amelioration. In the absence of infection, phenazopyridine (Pyridium) is appropriate for dysuria, oxybutynin (Ditropan) for urinary urgency, and flavoxate for bladder spasm.36
Sexual Dysfunction
Sexual dysfunction, including impotence, is common following radiation therapy for prostate cancer and, to a lesser extent, colorectal malignancies. Assessment of the true incidence is difficult, complicated by the comorbidities often found in older patients, who are most likely to be diagnosed with prostate cancer. Erectile dysfunction is more common with brachytherapy than with external beam radiation.40 Combined treatment protocols with radiation and total androgen ablation, such as with leuprolide (Lupron) and bicalutamide (Casodex), are synergistic in causing erectile dysfunction. Associated bladder and bowel dysfunction often lead to decreased intimacy and self-esteem.
Initially after radiation, few men experience erectile dysfunction. However, erectile function begins to decline 12 months after treatment and levels off at two years posttreatment. One study of men who had normal erectile function before radiation therapy found that 38 percent of men had erectile dysfunction one year after treatment and 59 percent after two years.41 The same study found a higher rate of posttreatment erectile dysfunction in men who had pretreatment erectile dysfunction.41 Phosphodiesterase type 5 inhibitors, such as sildenafil (Viagra) and tadalafil (Cialis), are effective for radiation-associated erectile dysfunction.42–44
Female sexual dysfunction is most common after radiation therapy for cervical and endometrial cancer. Adverse effects include decreased sexual interest, vaginal dryness and stenosis, dyspareunia, and general sexual dissatisfaction.59 There is some evidence to support the use of vaginal lubricating creams for vaginal irritation following radiation.47 Women older than 50 years are at higher risk of vaginal stenosis.46 The American Cancer Society recommends intercourse or use of a vaginal dilator three times per week to prevent stenosis.60
Men and women, as well as their spouses, may benefit from counseling or referral to a sex therapist or support group. Sexual counseling before treatment is also advised.45 Sperm or egg preservation should be discussed with patients considering posttreatment pregnancy. Ovarian transposition is an option for younger women requiring pelvic irradiation.48 Because of the teratogenicity of radiation, a pretreatment pregnancy test and birth control are essential. Hormonal contraception is contraindicated in patients with breast cancer and is a relative contraindication with several other malignancies. A male condom with spermicide provides a high rate of birth control, protection against sexually transmitted infections, and containment of seminal and vaginal secretions that may contain cytotoxic drugs.61
