
Am Fam Physician. 2010;82(5):488-493
Patient information: See related handout on prenatal radiation exposure, written by the authors of this article.
Author disclosure: Nothing to disclose.
Pregnant women are at risk of exposure to nonionizing and ionizing radiation resulting from necessary medical procedures, workplace exposure, and diagnostic or therapeutic interventions before the pregnancy is known. Nonionizing radiation includes microwave, ultrasound, radio frequency, and electromagnetic waves. In utero exposure to nonionizing radiation is not associated with significant risks; therefore, ultrasonography is safe to perform during pregnancy. Ionizing radiation includes particles and electromagnetic radiation (e.g., gamma rays, x-rays). In utero exposure to ionizing radiation can be teratogenic, carcinogenic, or mutagenic. The effects are directly related to the level of exposure and stage of fetal development. The fetus is most susceptible to radiation during organogenesis (two to seven weeks after conception) and in the early fetal period (eight to 15 weeks after conception). Noncancer health effects have not been detected at any stage of gestation after exposure to ionizing radiation of less than 0.05 Gy (5 rad). Spontaneous abortion, growth restriction, and mental retardation may occur at higher exposure levels. The risk of cancer is increased regardless of the dose. When an exposure to ionizing radiation occurs, the total fetal radiation dose should be estimated and the mother counseled about the potential risks so that she can make informed decisions about her pregnancy management.
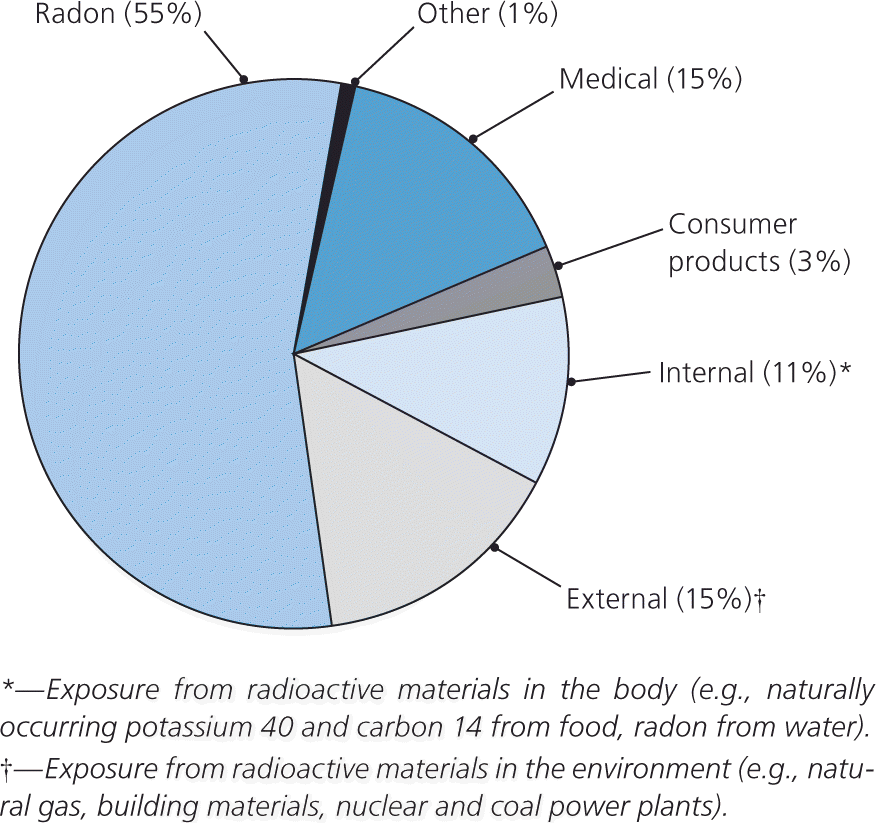
Physicians are sometimes asked to determine if prenatal exposure to radiation poses a risk for a developing fetus. All persons are regularly exposed to radiation, such as naturally occurring environmental radiation and radiation from industrial, occupational, and medical sources (Figure 1).1 Among pregnant women, the use of imaging tests requiring ionizing radiation increased 121 percent from 1997 to 2006, and the rate of computed tomography (CT) use increased by 25 percent per year.2 Lack of knowledge about the health consequences of radiation exposure may lead to unwarranted concern.3,4 Physicians who care for pregnant women often overestimate the teratogenic risk associated with diagnostic imaging.4,5 Knowledge of the potential effects of radiation allows physicians to provide appropriate counseling and medical decision making.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Pregnant women should be counseled that radiation exposure from a single diagnostic imaging procedure does not increase the risk of fetal anomalies or pregnancy loss. | C | 6, 16, 34 |
| Ultrasonography may be performed safely during pregnancy when it is medically indicated and when the lowest possible exposure setting is used. | C | 12–14 |
| A subspecialist in dosimetry should be consulted for calculation of the estimated fetal radiation dose when a patient is undergoing multiple diagnostic procedures or is exposed to internal radiation, or in whom a radiotherapeutic intervention is planned. | C | 6, 16 |
Forms of Radiation
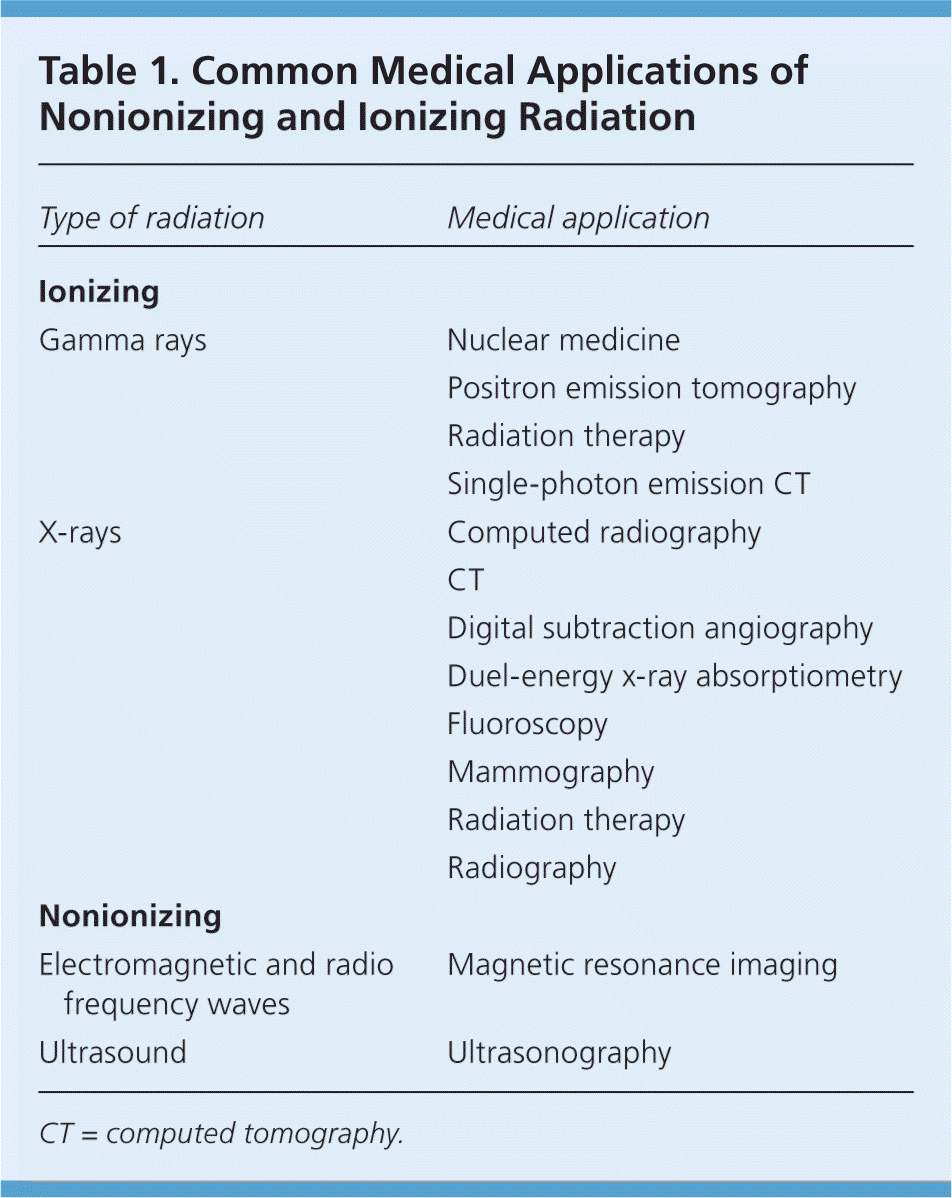
Radiation is fast-moving energy emitted as particles or waves. It is commonly divided into two categories: nonionizing and ionizing radiation. Nonionizing radiation is low-frequency radiation that disperses energy through heat and increased molecular movement. It includes visible light; ultraviolet rays; and microwave, ultrasound, radio frequency, and some electromagnetic waves. Ionizing radiation, which includes particles (alpha and beta particles) and some electromagnetic radiation (gamma rays and x-rays), can directly and indirectly alter the normal structure of a living cell.5 The average American is exposed to approximately 0.001 Gy (0.1 rad) of radiation annually from cosmic rays, radioactive substances in the environment, and naturally occurring radiation in the human body.1,6 Common medical applications of nonionizing and ionizing radiation are listed in Table 1.
| Type of radiation | Medical application |
|---|---|
| Ionizing | |
| Gamma rays | Nuclear medicine |
| Positron emission tomography | |
| Radiation therapy | |
| Single-photon emission CT | |
| X-rays | Computed radiography |
| CT | |
| Digital subtraction angiography | |
| Duel-energy x-ray absorptiometry | |
| Fluoroscopy | |
| Mammography | |
| Radiation therapy | |
| Radiography | |
| Nonionizing | |
| Electromagnetic and radio frequency waves | Magnetic resonance imaging |
| Ultrasound | Ultrasonography |
NONIONIZING RADIATION
Nonionizing radiation commonly interacts with tissue through the generation of heat. The impact of in utero exposure to non-ionizing radiation has been studied extensively, and no substantial risks have been consistently identified.7,8 Prenatal exposure to low-frequency electromagnetic fields has been of particular concern because of pooled epidemiologic evidence showing an association with childhood leukemia.9 Studies have not consistently found a causal link between prenatal exposure to electromagnetic fields and birth defects, miscarriage, or childhood leukemia.7,8,10,11
The most common type of diagnostic nonionizing radiation encountered in pregnancy is ultrasound. Bioeffect studies have not substantiated a causal relationship between prenatal ultrasonography and adverse fetal effects (e.g., childhood malignancies, birth weight, neurologic development).12,13 However, because of the potential for thermal and nonthermal tissue effects, ultrasonography should be performed only when medically indicated and using the lowest possible exposure setting under the ALARA (as low as reasonably achievable) principle.14,15
Magnetic resonance imaging (MRI) is emerging as another form of medical nonionizing radiation for use during pregnancy, especially when ultrasound findings are indeterminiate.16 Studies of children with in utero exposure to MRI at 1.5 tesla have not demonstrated any negative outcomes at up to nine years of age.17,18
IONIZING RADIATION
Ionizing radiation acts directly with the biochemical structures in tissue (including proteins, DNA, and other molecules) or indirectly by causing the formation of free radicals, which in turn break the structure of proteins, DNA, or any other critical part of the cell. The effects of exposure may be classified as deterministic or stochastic.19
Deterministic effects result from radiation-induced cellular injury or death and are characterized by a threshold dose. For a given dose of radiation (the threshold dose), there is loss of organ or tissue functionality, leading to pregnancy loss, congenital malformations, neurobehavioral abnormalities, and fetal growth restriction. The severity and the incidence of these effects increase with the dose. Below the threshold dose, there seems to be no risk of these effects.19,20
Stochastic effects are caused by radiation-induced changes in cells that maintain their capability for replication. Over time, these cells can become malignant. Unlike deterministic effects, stochastic effects do not seem to have a threshold dose. Their probability depends on the radiation dose and follows a simple, linear, no-threshold model, which is the basis for current radiation protection standards and practices.21 The hypothesis of this model is based largely on humans exposed to high doses and dose rates, and postulates that any exposure to ionizing radiation may be harmful.
Risks of Ionizing Radiation During Pregnancy
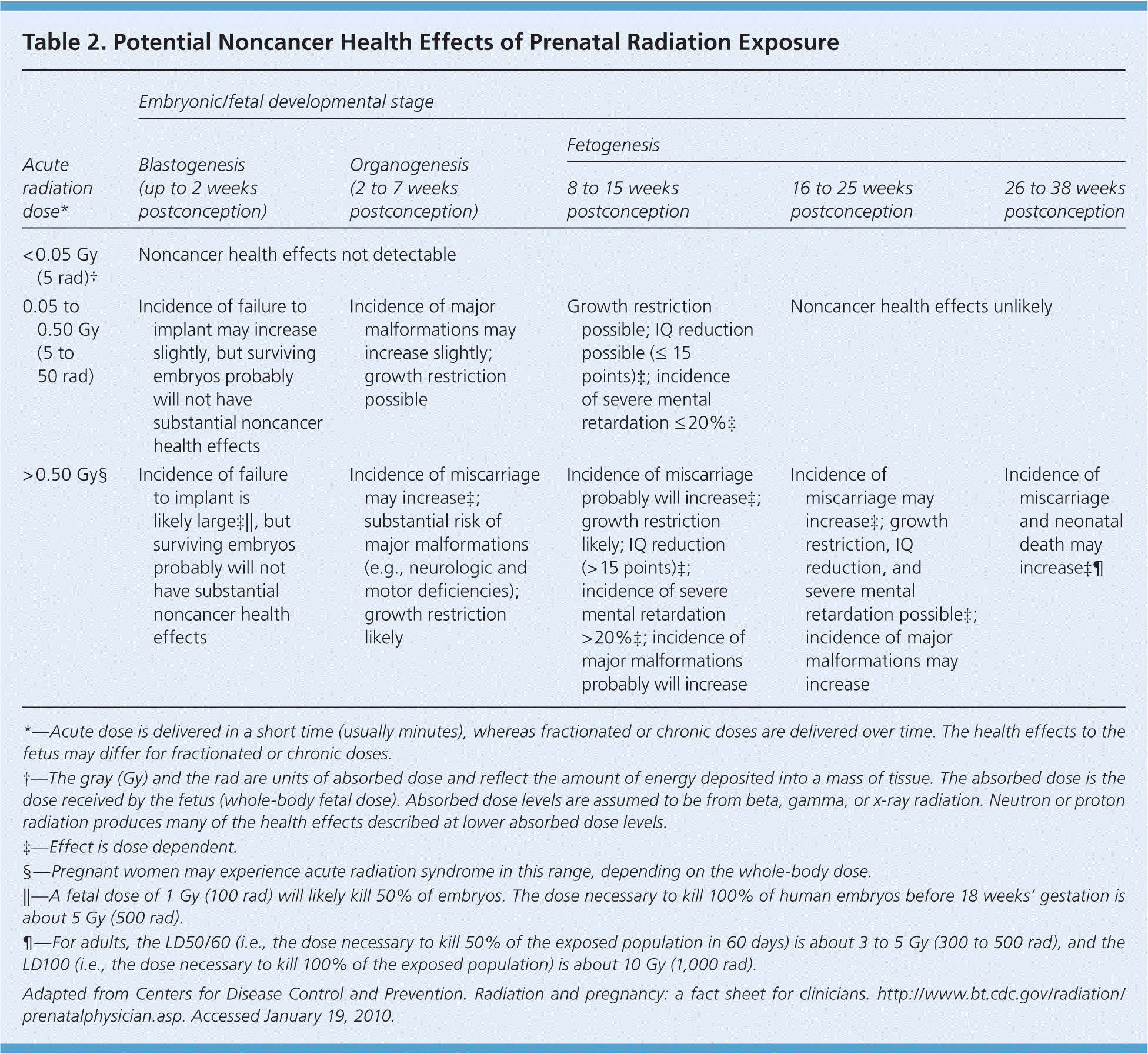
The risks of prenatal exposure to ionizing radiation vary based on the stage of development22–24 (Table 2).24 An embryo is most susceptible to the effects of radiation during organogenesis (two to seven weeks after conception) and in the early fetal period (eight to 15 weeks after conception).6 The effects of exposure can be teratogenic, carcinogenic, or mutagenic and are directly related to the level of radiation exposure.6 Risk estimates for teratogenic (noncancer) and carcinogenic effects have been calculated in atomic bomb and nuclear fallout survivors, early use of diagnostic imaging in pregnant women, and animal studies.
| Acute radiation dose* | Embryonic/fetal developmental stage | ||||
|---|---|---|---|---|---|
| Blastogenesis (up to 2 weeks postconception) | Organogenesis (2 to 7 weeks postconception) | Fetogenesis | |||
| 8 to 15 weeks postconception | 16 to 25 weeks postconception | 26 to 38 weeks postconception | |||
| < 0.05 Gy (5 rad)† | Noncancer health effects not detectable | ||||
| 0.05 to 0.50 Gy (5 to 50 rad) | Incidence of failure to implant may increase slightly, but surviving embryos probably will not have substantial noncancer health effects | Incidence of major malformations may increase slightly; growth restriction possible | Growth restriction possible; IQ reduction possible (≤ 15 points)‡; incidence of severe mental retardation ≤20%‡ | Noncancer health effects unlikely | |
| > 0.50 Gy§ | Incidence of failure to implant is likely large‡∥, but surviving embryos probably will not have substantial noncancer health effects | Incidence of miscarriage may increase‡; substantial risk of major malformations (e.g., neurologic and motor deficiencies); growth restriction likely | Incidence of miscarriage probably will increase‡; growth restriction likely; IQ reduction (> 15 points)‡; incidence of severe mental retardation >20%‡; incidence of major malformations probably will increase | Incidence of miscarriage may increase‡; growth restriction, IQ reduction, and severe mental retardation possible‡; incidence of major malformations may increase | Incidence of miscarriage and neonatal death may increase‡¶ |
Noncancer health effects are not observed when the fetal radiation exposure occurs below a threshold dose of 0.05 Gy (5 rad) at any stage of gestation.6 In clinical practice, the threshold dose of the human embryo is probably closer to 0.10 to 0.20 Gy (10 to 20 rad).20,25 After 16 weeks postconception, the threshold dose for non-cancer effects increases to approximately 0.50 to 0.70 Gy (50 to 70 rad).20,25
During the preimplantation period (blastogenesis), exposure to a radiation dose greater than 0.1 Gy (10 rad) is associated with a risk of failure to implant, representing an “all or none” phenomenon of early embryonic development. If the embryo survives, substantial noncancer health effects are unlikely, regardless of the radiation dose.21,23,25 Mental retardation and growth restriction, including microcephaly, are the most common fetal malformations after significant radiation exposure during organogenesis and the early fetal period.21,22,26–29 Atomic bomb survivors who received in utero doses greater than 1 Gy (100 rad) had a permanent 3 to 4 percent reduction in height at 18 years of age.27 Studies of Japanese atomic bomb survivors have shown that a threshold dose of 0.3 Gy (30 rad) at eight to 15 weeks after conception is associated with an increased risk of severe mental retardation.28,29 IQ data in this cohort fit a linear dose-response model, with an average IQ loss of approximately 25 to 31 points per 1 Gy beyond 0.1 Gy.30
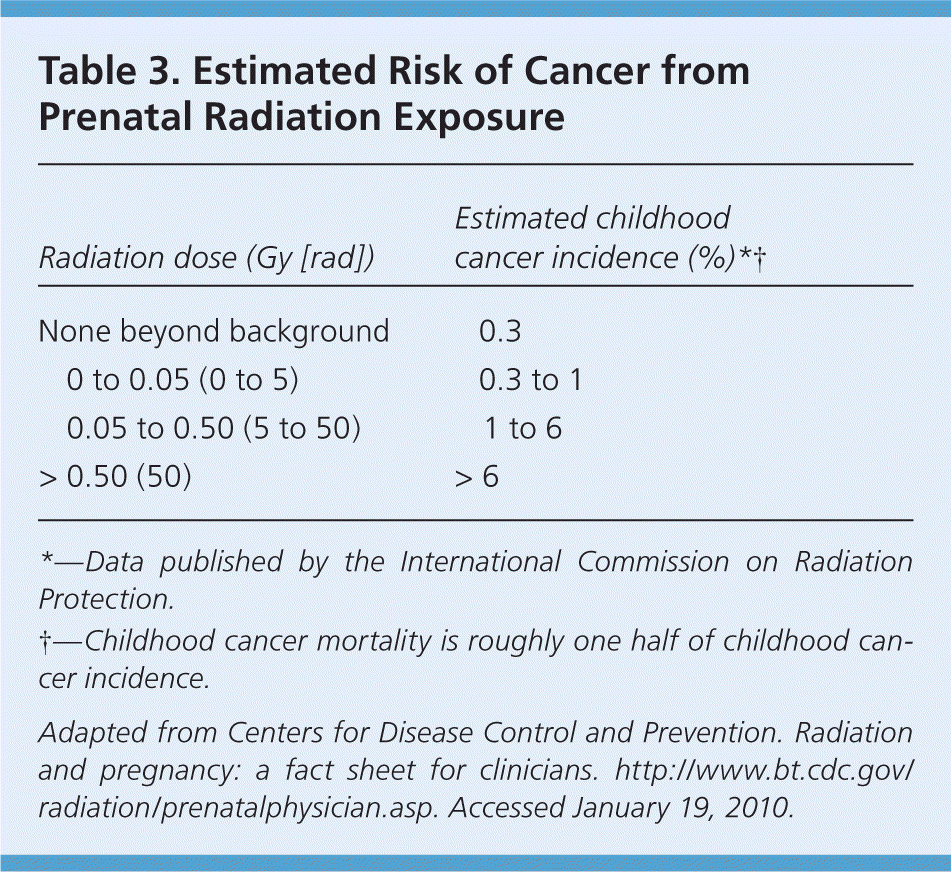
In utero exposure to ionizing radiation at any dose is associated with an increased risk of childhood malignancy, especially leukemia.31,32 The risk of childhood cancer after prenatal radiation exposure has been estimated (Table 3).24 Although lifetime cancer risk is unknown, there is increasing evidence of elevated risk, particularly for solid tumors.33 A systematic review of epidemiologic studies did not find an increased risk of childhood cancer after in utero exposure to diagnostic x-rays.34 However, the authors advise that their findings be interpreted with caution because of limitations in the quality of the reviewed studies and the very low exposure doses.
Caring for Patients with Prenatal Radiation Exposure
Although avoiding radiation in pregnant women is preferred, exposure may occur for three common reasons: medical necessity, unknown pregnancy at the time of diagnostic or therapeutic intervention, and workplace exposure to ionizing radiation.
When an unanticipated exposure has occurred or a diagnostic or therapeutic intervention is planned, the first step is to estimate the cumulative radiation dose to the fetus. Approximate fetal radiation doses for common radiologic procedures are given in Table 4.2,4,16 Complex calculations are required for internal radiation doses because substances needed for fetal growth and development (e.g., iodine) may concentrate in fetal tissue, whereas radioactive substances may concentrate in maternal tissues surrounding the uterus. Under these circumstances, consultation with a hospital medical physicist or health physicist is recommended.6,16 Listings for active, certified health physicists are available through the Health Physics Society at http://www.hps1.org/aahp/members/members.htm.
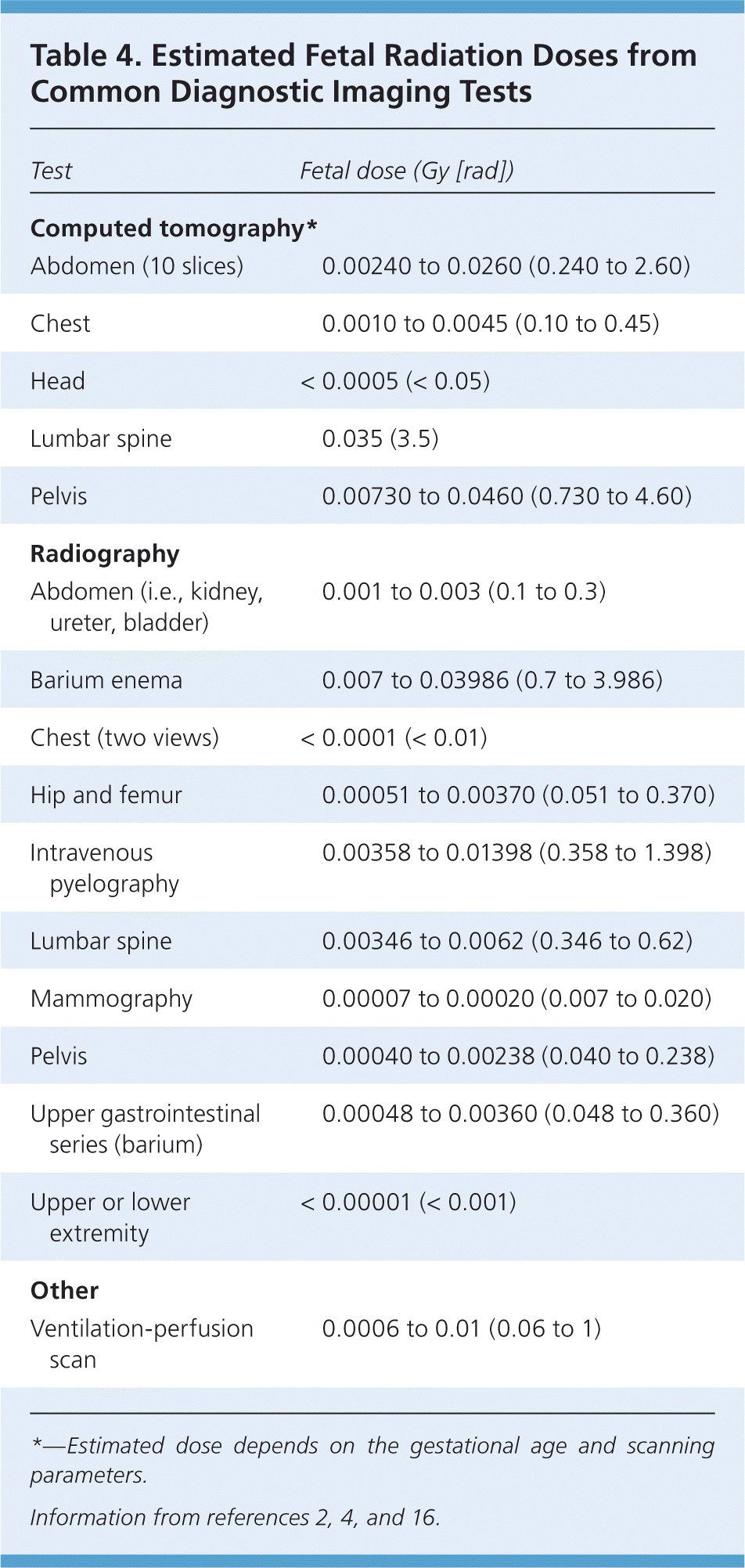
| Test | Fetal dose (Gy [rad]) |
|---|---|
| Computed tomography* | |
| Abdomen (10 slices) | 0.00240 to 0.0260 (0.240 to 2.60) |
| Chest | 0.0010 to 0.0045 (0.10 to 0.45) |
| Head | < 0.0005 (< 0.05) |
| Lumbar spine | 0.035 (3.5) |
| Pelvis | 0.00730 to 0.0460 (0.730 to 4.60) |
| Radiography | |
| Abdomen (i.e., kidney, ureter, bladder) | 0.001 to 0.003 (0.1 to 0.3) |
| Barium enema | 0.007 to 0.03986 (0.7 to 3.986) |
| Chest (two views) | < 0.0001 (< 0.01) |
| Hip and femur | 0.00051 to 0.00370 (0.051 to 0.370) |
| Intravenous pyelography | 0.00358 to 0.01398 (0.358 to 1.398) |
| Lumbar spine | 0.00346 to 0.0062 (0.346 to 0.62) |
| Mammography | 0.00007 to 0.00020 (0.007 to 0.020) |
| Pelvis | 0.00040 to 0.00238 (0.040 to 0.238) |
| Upper gastrointestinal series (barium) | 0.00048 to 0.00360 (0.048 to 0.360) |
| Upper or lower extremity | < 0.00001 (< 0.001) |
| Other | |
| Ventilation-perfusion scan | 0.0006 to 0.01 (0.06 to 1) |
Once the fetal radiation dose is estimated, the potential health effects can be assessed. Discussions with patients should include the immediate and short-term risks to the fetus, as well as the estimated risk of the child developing cancer later in life.24 It may be helpful to compare this information with baseline risks of pregnancy complications without exposure (e.g., 15 percent risk of spontaneous abortion, 2 to 4 percent incidence of major malformation, 4 percent incidence of intrauterine growth restriction, 9 to 10 percent risk of genetic defects).6 The use of corollary statements is also helpful (e.g., “A fetal radiation dose of 0.05 Gy has a small risk of radiation-induced cancer. There is approximately a 99 percent chance that the exposed fetus will not develop childhood cancer or leukemia.”). After an objective discussion of these risk estimates, pregnant women should be counseled in a nondirective manner and encouraged to make an informed decision about their pregnancy management.
Safety Counseling
All pregnant women are entitled to counseling before exposure to radiation.6,35 The extent of communication about the effects of radiation should be related to the level of risk. A detailed explanation should be provided if the anticipated fetal dose or actual exposure exceeds 0.01 Gy (1 rad).6 Women should be counseled that under most circumstances, diagnostic radiography during pregnancy is safe, and that radiation exposure from a single diagnostic imaging procedure has not been associated with an increase in fetal anomalies or pregnancy loss.16,24,35 Exposure to a cumulative dose of less than 0.05 Gy—equivalent to the radiation dose from approximately 500 chest radiographs or 100 abdominal CT scans—has not been shown to affect pregnancy outcomes compared with control populations exposed to background radiation.5,6,20 However, alternative imaging procedures (e.g., ultrasonography, MRI) should be considered when appropriate.16
Occupational Considerations
Most states have laws that guide organizations in protecting the health and safety of pregnant employees who work in areas where radiation is used. Rules for radiation dose limits apply once a pregnant employee declares her pregnancy. The Code of Federal Regulations sets an occupational exposure limit of 0.005 Gy (0.5 rad) for women who have declared a pregnancy.36 Employers must attempt to maintain uniform monthly exposure throughout the pregnancy. Dose monitoring is recommended if a pregnant woman may be exposed to an excess dose.
Additional Resources
The Centers for Disease Control and Prevention (CDC) provides online resources regarding radiation exposures (http://www.bt.cdc.gov/radiation/clinicians.asp). The CDC's fact sheet on prenatal radiation exposure is helpful when counseling pregnant patients who have been exposed to radiation.24
