
Am Fam Physician. 2010;82(11):1381-1388
Author disclosure: Nothing to disclose.
Careful examination of the oral cavity may reveal findings indicative of an underlying systemic condition, and allow for early diagnosis and treatment. Examination should include evaluation for mucosal changes, periodontal inflammation and bleeding, and general condition of the teeth. Oral findings of anemia may include mucosal pallor, atrophic glossitis, and candidiasis. Oral ulceration may be found in patients with lupus erythematosus, pemphigus vulgaris, or Crohn disease. Additional oral manifestations of lupus erythematosus may include honeycomb plaques (silvery white, scarred plaques); raised keratotic plaques (verrucous lupus erythematosus); and nonspecific erythema, purpura, petechiae, and cheilitis. Additional oral findings in patients with Crohn disease may include diffuse mucosal swelling, cobblestone mucosa, and localized mucogingivitis. Diffuse melanin pigmentation may be an early manifestation of Addison disease. Severe periodontal inflammation or bleeding should prompt investigation of conditions such as diabetes mellitus, human immunodeficiency virus infection, thrombocytopenia, and leukemia. In patients with gastroesophageal reflux disease, bulimia, or anorexia, exposure of tooth enamel to acidic gastric contents may cause irreversible dental erosion. Severe erosion may require dental restorative treatment. In patients with pemphigus vulgaris, thrombocytopenia, or Crohn disease, oral changes may be the first sign of disease.
In 2000, the U.S. Surgeon General's report Oral Health in America highlighted numerous ways in which oral and general health are linked.1 Oral examination can reveal signs and symptoms of immunologic diseases, endocrinopathies, hematologic conditions, systemic infections, and nutritional disorders. In addition, several studies have reported associations between periodontal disease and diabetes mellitus, heart disease, stroke, and adverse pregnancy outcomes.2–4 Identifying these oral findings may allow for early diagnosis and treatment. Family physicians should be familiar with the relationship between systemic and oral health, and be prepared to coordinate care with dental or medical subspecialists as indicated.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| In patients with Crohn disease, oral lesions that persist despite systemic treatment of underlying intestinal disease may respond to topical or intralesionally injected corticosteroids. | C | 16–18 | Based on case series and usual practice |
| Treatment of periodontitis in patients with diabetes mellitus can lead to improved glycemic control. | B | 23–28 | Findings based on two RCTs, three meta-analyses, and one uncontrolled comparison study |
| Improvement in glycemic control found in all studies, although not all reached statistical significance | |||
| Various preventive protocols (e.g., acyclovir [Zovirax], nystatin, chlorhexidine [Peridex], oral hygiene care) may be considered to minimize secondary oral opportunistic infection and chemotherapy-related oral mucositis in patients receiving treatment for leukemia. | B | 33–38 | Based on one meta-analysis of herpes simplex virus prevention with acyclovir; two meta-analyses of mucositis prevention with chlorhexidine; one RCT and one randomized crossover trial examining mucositis prevention with chlorhexidine and oral care; and one RCT examining mucositis and candidiasis prevention with chlorhexidine, oral hygiene care, iodopovidone, and nystatin |
This article provides a guide for recognizing the oral manifestations of select systemic diseases. A number of oral manifestations of systemic disease have been covered previously5,6; therefore, a detailed discussion of these findings is not provided here. However, for comprehensiveness, salient features of these findings are included in Table 1, with a summary of the conditions and associated oral manifestations discussed in this article. For each category of oral finding, the conditions are presented in order of the frequency in which oral manifestations are encountered, from the most to least common.
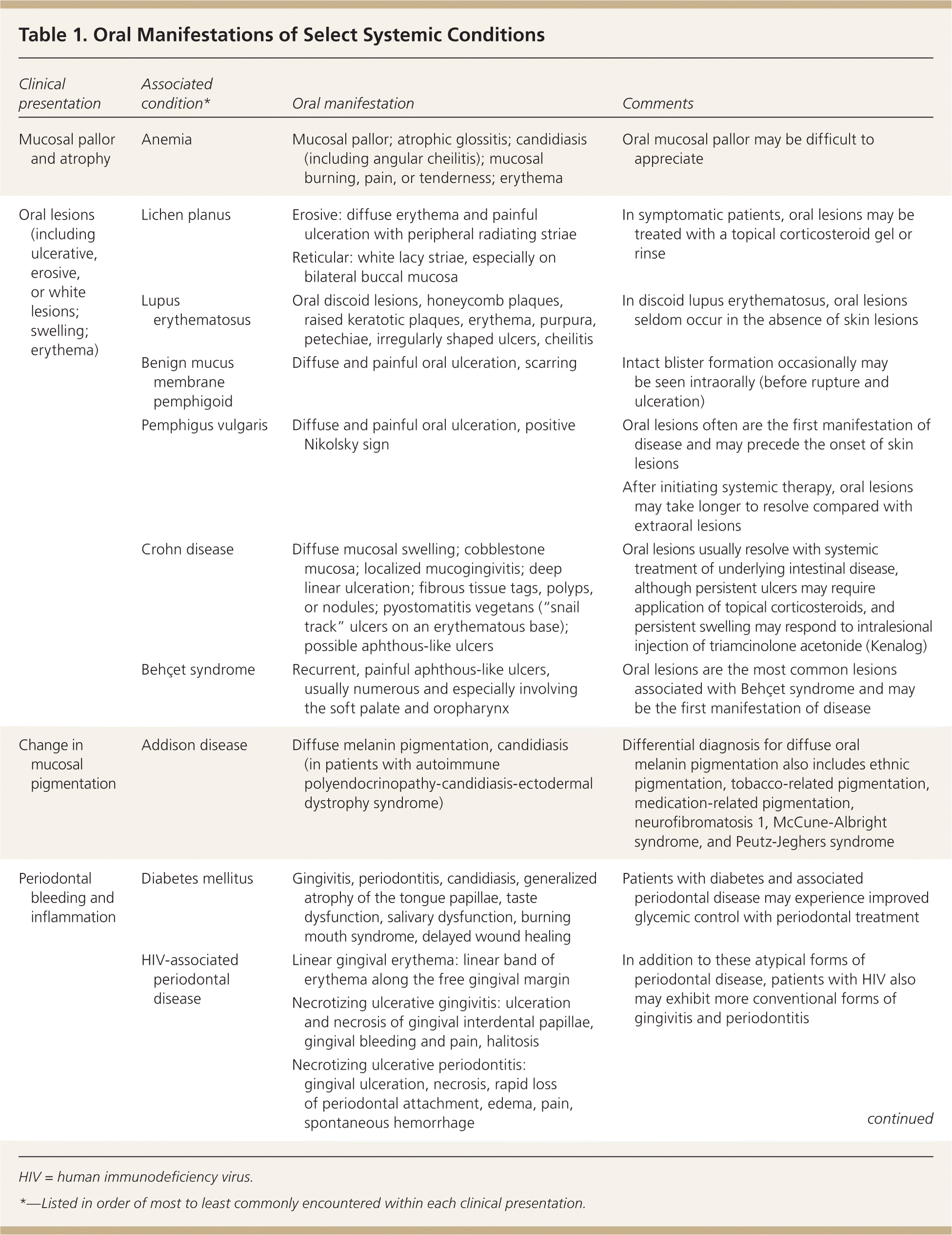
| Clinical presentation | Associated condition* | Oral manifestation | Comments |
|---|---|---|---|
| Mucosal pallor and atrophy | Anemia | Mucosal pallor; atrophic glossitis; candidiasis (including angular cheilitis); mucosal burning, pain, or tenderness; erythema | Oral mucosal pallor may be difficult to appreciate |
| Oral lesions (including ulcerative, erosive, or white lesions; swelling; erythema) | Lichen planus | Erosive: diffuse erythema and painful ulceration with peripheral radiating striae | In symptomatic patients, oral lesions may be treated with a topical corticosteroid gel or rinse |
| Reticular: white lacy striae, especially on bilateral buccal mucosa | |||
| Lupus erythematosus | Oral discoid lesions, honeycomb plaques, raised keratotic plaques, erythema, purpura, petechiae, irregularly shaped ulcers, cheilitis | In discoid lupus erythematosus, oral lesions seldom occur in the absence of skin lesions | |
| Benign mucus membrane pemphigoid | Diffuse and painful oral ulceration, scarring | Intact blister formation occasionally may be seen intraorally (before rupture and ulceration) | |
| Pemphigus vulgaris | Diffuse and painful oral ulceration, positive Nikolsky sign | Oral lesions often are the first manifestation of disease and may precede the onset of skin lesions | |
| After initiating systemic therapy, oral lesions may take longer to resolve compared with extraoral lesions | |||
| Crohn disease | Diffuse mucosal swelling; cobblestone mucosa; localized mucogingivitis; deep linear ulceration; fibrous tissue tags, polyps, or nodules; pyostomatitis vegetans (“snail track” ulcers on an erythematous base); possible aphthous-like ulcers | Oral lesions usually resolve with systemic treatment of underlying intestinal disease, although persistent ulcers may require application of topical corticosteroids, and persistent swelling may respond to intralesional injection of triamcinolone acetonide (Kenalog) | |
| Behçet syndrome | Recurrent, painful aphthous-like ulcers, usually numerous and especially involving the soft palate and oropharynx | Oral lesions are the most common lesions associated with Behçet syndrome and may be the first manifestation of disease | |
| Change in mucosal pigmentation | Addison disease | Diffuse melanin pigmentation, candidiasis (in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome) | Differential diagnosis for diffuse oral melanin pigmentation also includes ethnic pigmentation, tobacco-related pigmentation, medication-related pigmentation, neurofibromatosis 1, McCune-Albright syndrome, and Peutz-Jeghers syndrome |
| Periodontal bleeding and inflammation | Diabetes mellitus | Gingivitis, periodontitis, candidiasis, generalized atrophy of the tongue papillae, taste dysfunction, salivary dysfunction, burning mouth syndrome, delayed wound healing | Patients with diabetes and associated periodontal disease may experience improved glycemic control with periodontal treatment |
| HIV-associated periodontal disease | Linear gingival erythema: linear band of erythema along the free gingival margin | In addition to these atypical forms of periodontal disease, patients with HIV also may exhibit more conventional forms of gingivitis and periodontitis | |
| Necrotizing ulcerative gingivitis: ulceration and necrosis of gingival interdental papillae, gingival bleeding and pain, halitosis | |||
| Necrotizing ulcerative periodontitis: gingival ulceration, necrosis, rapid loss of periodontal attachment, edema, pain, spontaneous hemorrhage | |||
| Thrombocytopenia | Petechiae, purpura, ecchymosis, hemorrhagic bullae, hematomas | Hemorrhage may occur with minor trauma or spontaneously | |
| Leukemia | Mucosal bleeding, ulceration, petechiae, and diffuse or localized gingival enlargement; secondary infections (e.g., candidiasis, herpes simplex virus infection, periodontal bone loss) | Gingival infiltration by leukemic cells occurs most often in acute monocytic leukemia and acute myelomonocytic leukemia | |
| Dental erosion | Gastroesophageal reflux disease | Water brash, xerostomia, burning sensation, halitosis, palatal erythema, dental erosion | Dental erosion may require dental restorative treatment |
| Other oral findings usually will resolve with medical management of gastroesophageal reflex disease | |||
| Bulimia and anorexia | Dental erosion, xerostomia, increased caries rate, sialadenosis (especially bilateral parotid enlargement) | Dental erosion may require dental restorative treatment | |
| Xerostomia and sialadenosis usually resolve on normalization of nutritional status; sialogogues may help |
Mucosal Changes
MUCOSAL PALLOR AND ATROPHY
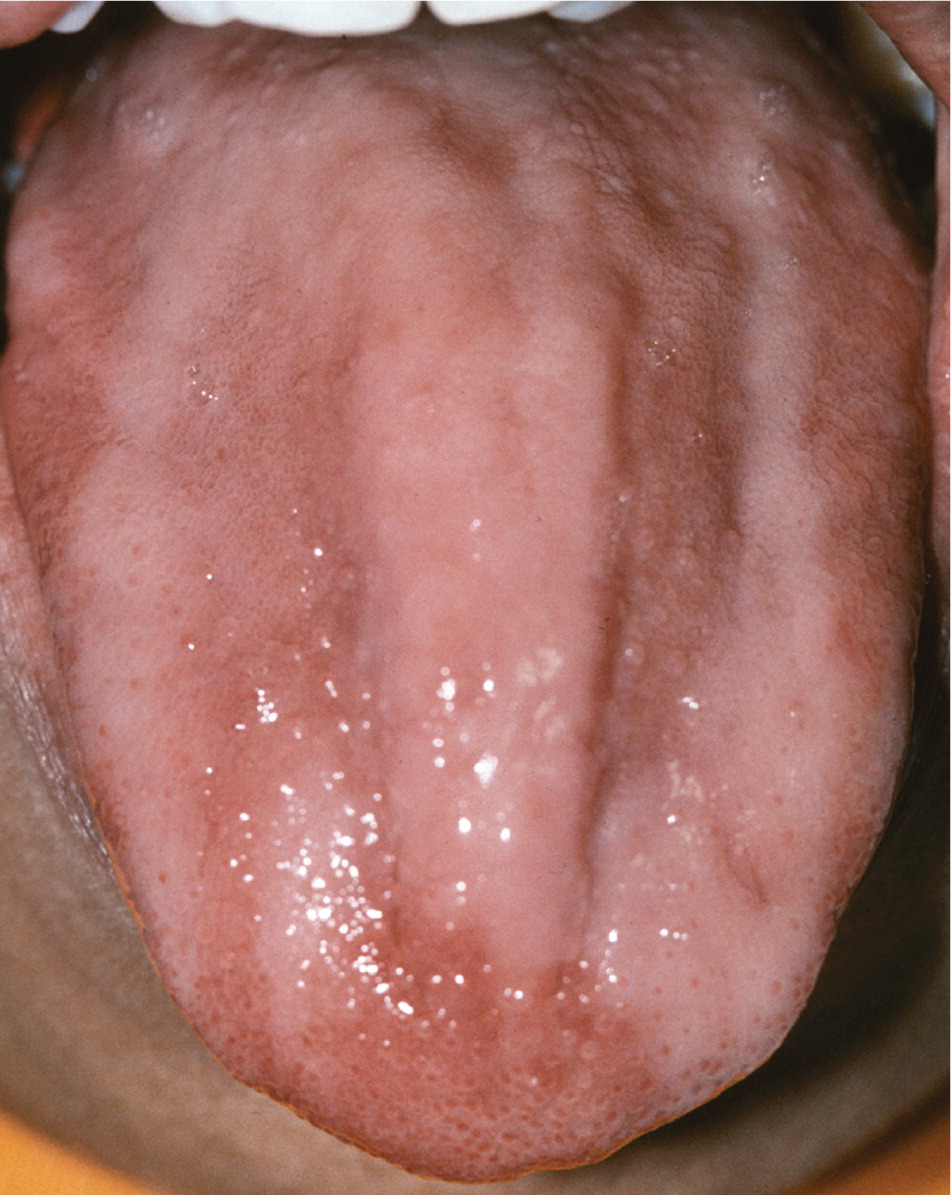
Oral findings in patients with anemia may include mucosal pallor, atrophic glossitis, and candidiasis. Oral mucosal pallor may be difficult to appreciate.7 Atrophic glossitis appears as complete or patchy baldness of the tongue caused by atrophy of the lingual papillae (Figure 1). Atrophic glossitis is a nonspecific finding that can occur in association with iron deficiency anemia, pernicious anemia/vitamin B complex deficiencies, and various other conditions. Atrophy can be observed most easily on the dorsal tongue, although other sites may be affected. Burning, pain, tenderness, and erythema also may be present. Candidiasis may be a concurrent finding or an alternative cause of erythema, burning, and atrophy. In addition, some patients may present with angular cheilitis (a lip infection caused by Candida albicans or Staphylococcus aureus), which appears as erythema, fissuring, and crusting at the corners of the mouth.
ORAL LESIONS
In addition to ulcerative or erosive lesions, white lesions or nonspecific erythema may be indicative of systemic disease.
Lupus erythematosus. The reported frequency of oral lesions ranges from 8 to 45 percent in patients with systemic lupus erythematosus and from 4 to 25 percent in patients with discoid lupus erythematosus.8 Descriptions of oral lesions in lupus erythematosus vary greatly. The classic presentation is the oral discoid lesion, characterized by a well-demarcated zone of erythema, atrophy, or ulceration surrounded by white, radiating striae. These lesions appear similar to those found in patients with erosive lichen planus.6 Variations include honeycomb plaques (silvery white, scarred plaques), raised keratotic plaques (verrucous lupus erythematosus; Figure 2), and nonspecific erythema9 (Figure 3). Purpura, petechiae, or irregularly shaped ulcers also are possible. Discoid lesions like those typically found elsewhere on sun-exposed skin may be found on the lip vermilion. Cheilitis may be evident as well.10
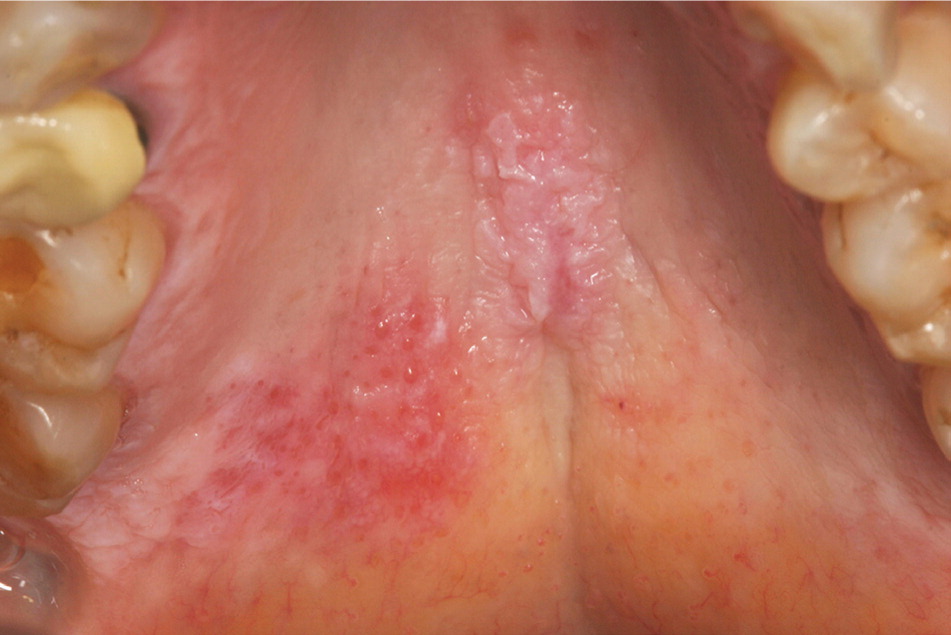
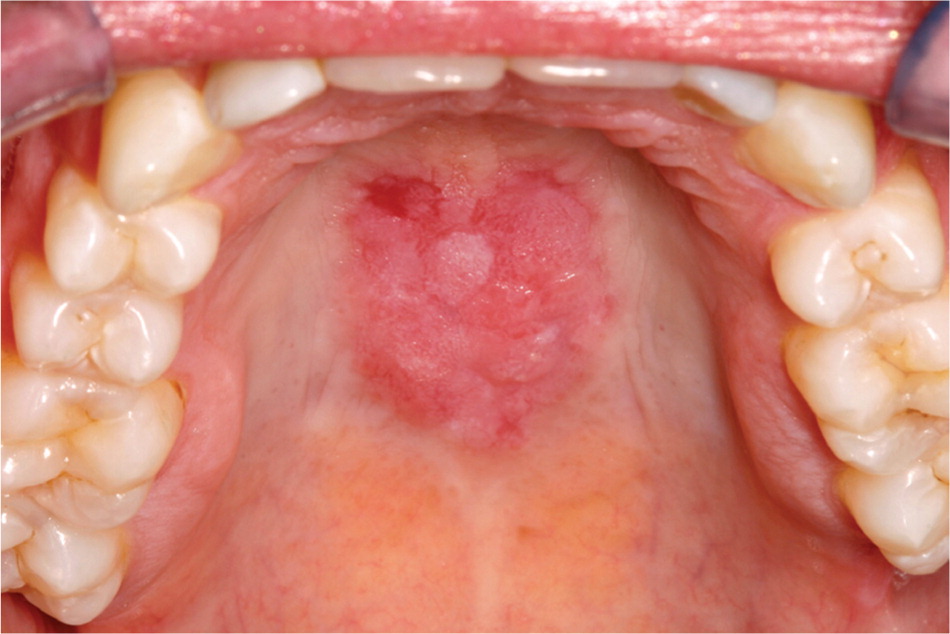
Oral lesions in patients with systemic lupus erythematosus typically resolve with systemic immunosuppressive therapy. For patients with limited skin or oral mucosal disease, topical corticosteroids or systemic antimalarial drugs are appropriate.11
Pemphigus vulgaris. Oral lesions are the initial manifestation in 50 to 80 percent of patients with pemphigus vulgaris, and may precede skin lesions by one year or more.12 Patients typically experience painful, diffuse oral ulceration (Figure 4). It is difficult to observe intact blisters intraorally because the lesions rupture easily. However, a positive Nikolsky sign (i.e., blister formation on normal-appearing mucosa or skin with application of firm lateral pressure) may be elicited. Extraoral findings may include cutaneous blisters, crusted skin erosions, and bilateral conjunctivitis. The differential diagnosis for chronic, multifocal oral ulceration includes other systemic immune-mediated conditions, such as erosive lichen planus and benign mucus membrane pemphigoid (Table 1).
Oral lesions usually resolve with systemic immunosuppressive therapy, but may be slower to resolve compared with extraoral lesions.
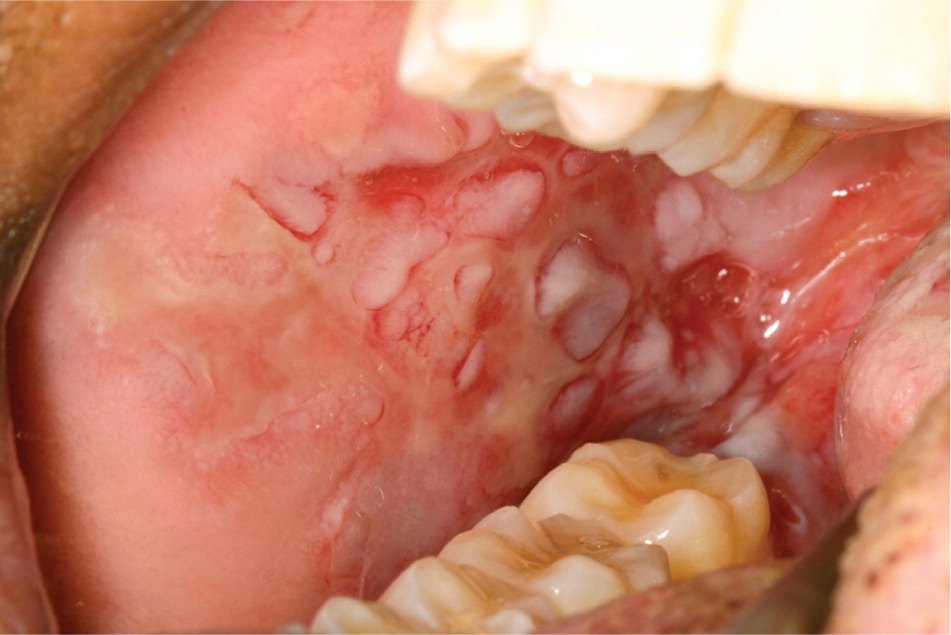
Crohn disease. The reported prevalence of oral lesions in Crohn disease ranges from 0.5 to 20 percent.13,14 Oral lesions may precede abdominal symptoms and do not necessarily correlate with intestinal disease activity15,16 (Table 1). Descriptions of oral lesions in Crohn disease vary widely. Some characteristic abnormalities include diffuse swelling, cobblestone appearance of the mucosa, localized mucogingivitis, and deep linear ulceration. The swelling usually is persistent, firm, and painless, and tends to involve the lips, buccal mucosa, and facial soft tissues. The deep linear ulcers often occur at the depth of the buccal vestibule and may be surrounded by hyperplastic margins (Figure 5). Secondary fibrosis can produce tissue tags, polyps, or nodules (Figure 6). An uncommon presentation is pyostomatitis vegetans, characterized by serpentine pustules that coalesce in a “snail track” pattern.
CHANGES IN PIGMENTATION
Hyperpigmentation of the oral mucosa (i.e., Addison disease) may be the first manifestation of primary adrenal insufficiency19 (Figure 7). However, diffuse melanin pigmentation of the oral mucosa is a nonspecific finding, and numerous other conditions may be considered in the differential diagnosis (e.g., ethnic pigmentation, tobacco-related pigmentation, medication-related pigmentation, neurofibromatosis 1, McCune-Albright syndrome, Peutz-Jeghers syndrome).
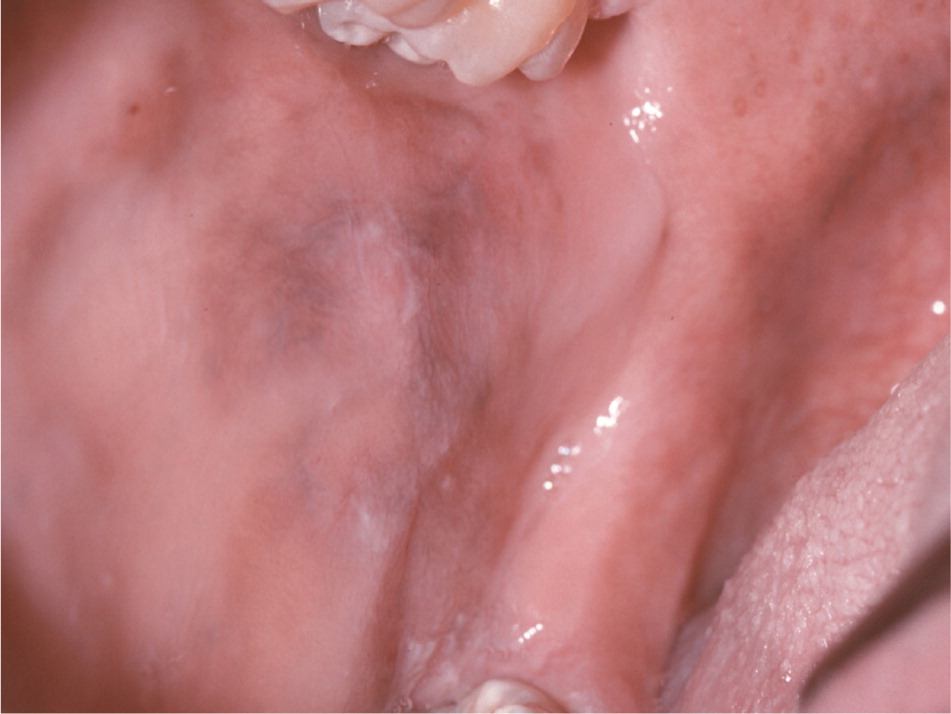
Primary adrenal insufficiency may occur in association with the autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome. In this condition, chronic mucocutaneous candidiasis develops in childhood in conjunction with hypoparathyroidism and other findings. In the oral cavity, candidiasis can present as pseudomembranous (white plaques that can be wiped away), hyperplastic (white plaques that cannot be wiped away), or erythematous.
Periodontal Bleeding and Inflammation
DIABETES
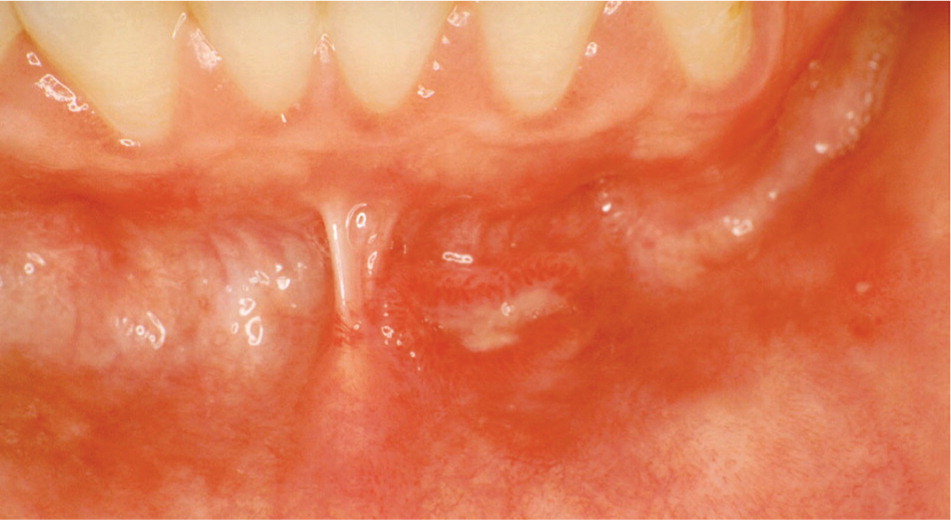
There is a strong association between diabetes and periodontal diseases, including gingivitis (gingival inflammation) and periodontitis (inflammation and destruction of the periodontal ligament and alveolar bone that hold teeth in place; Figure 8). Notably, there is emerging evidence of a two-way relationship—diabetes can lead to poor periodontal health, and poor periodontal health can make it difficult to control diabetes.20 Patients with poorly controlled diabetes experience significantly greater periodontal attachment loss compared with patients with well-controlled diabetes or those without diabetes.21,22 In addition, treatment for periodontitis may improve glycemic control; however, further studies are needed to confirm these findings.23–28 Furthermore, severe periodontal disease may be a strong predictor of various diabetic complications, including nephropathy, stroke, transient ischemic attack, angina, myocardial infarction, and heart failure.29,30 The International Diabetes Federation recommends that primary care for diabetes should include annually inquiring about symptoms of gum disease (e.g., bleeding when brushing, swollen or red gingiva) and encouraging regular evaluation and treatment by a dental health professional.31
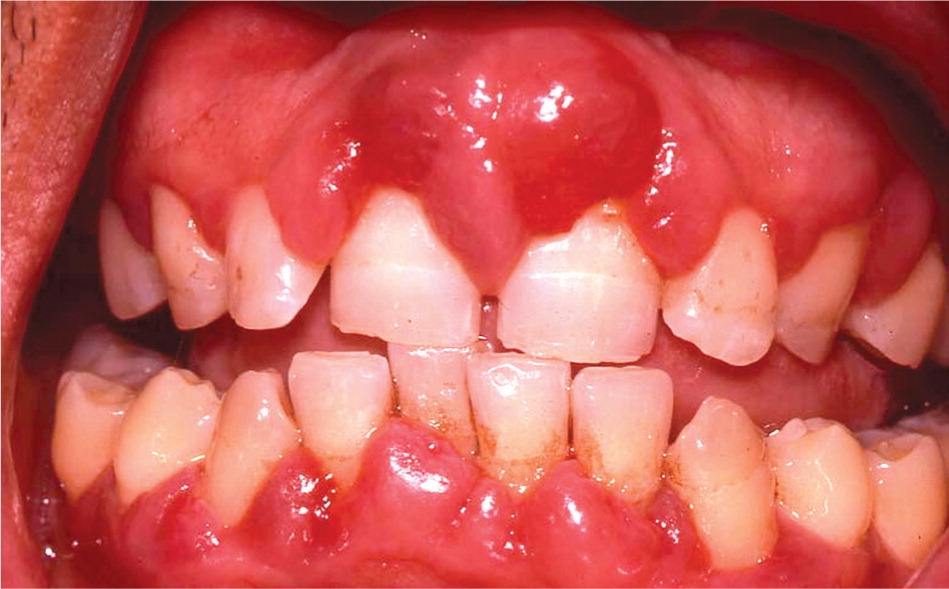
Additional oral or head and neck findings may include candidiasis, sialadenosis (bilateral noninflammatory enlargement of the parotid glands), generalized atrophy of the tongue papillae, taste dysfunction, salivary dysfunction, burning mouth syndrome, and delayed wound healing.
THROMBOCYTOPENIA
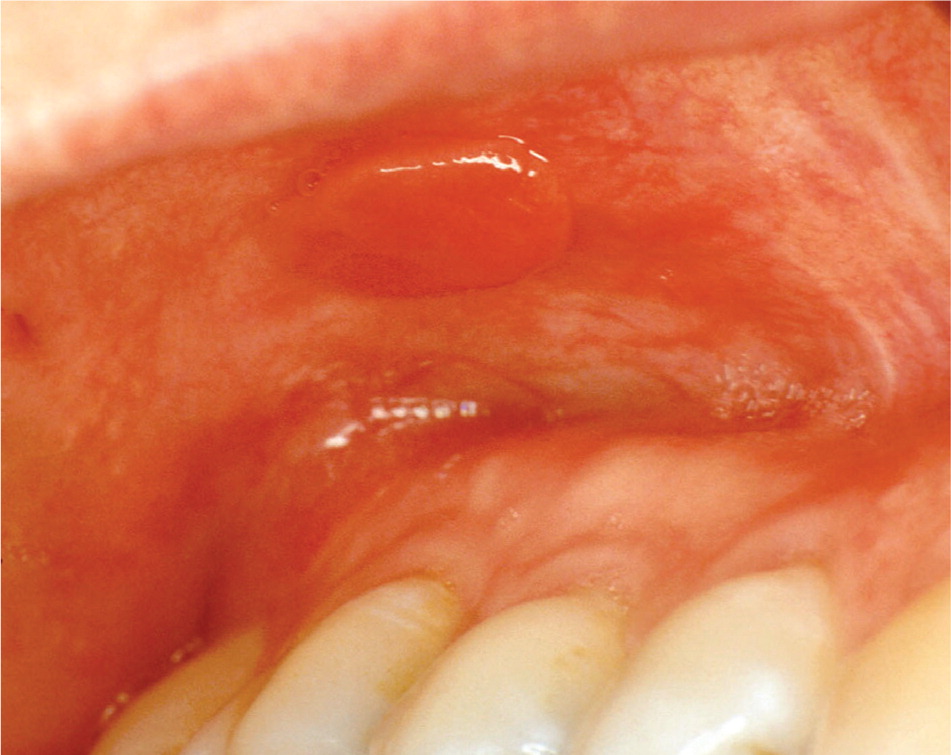
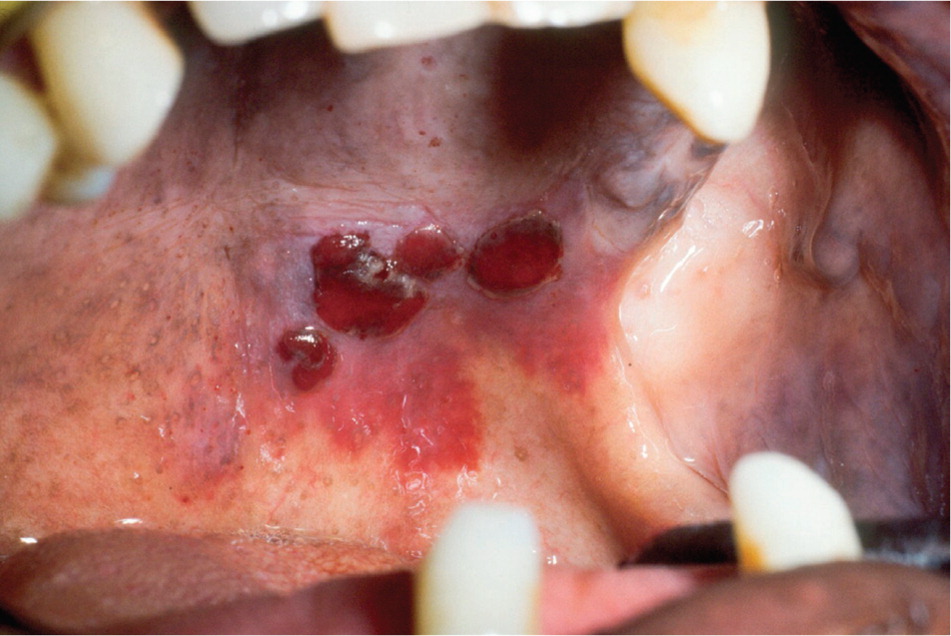
In many cases, thrombocytopenia (platelet count usually less than 50 × 103 per μL [50 × 109 per L]) may be detected initially because of oral lesion development. Minor trauma to the oral mucosa during routine function (such as chewing or swallowing) may produce various types of hemorrhagic lesions, including petechiae, purpura, ecchymosis, hemorrhagic bullae, and hematoma formation (Figure 9). In addition, gingival bleeding may result from minor trauma or occur spontaneously.
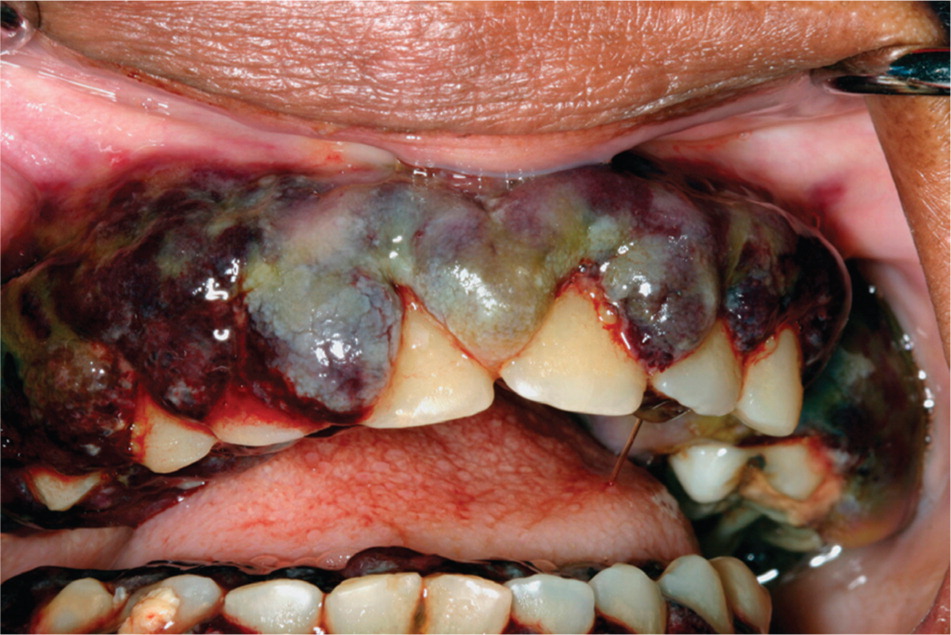
LEUKEMIA
Oral manifestations of leukemia may include mucosal bleeding, ulceration, petechiae, and diffuse or localized gingival enlargement (Figure 10). Gingival infiltration by leukemic cells occurs most often in acute monocytic leukemia and acute myelomonocytic leukemia.32 The gingiva may feel boggy and appear hemorrhagic with or without concurrent ulceration. Impaired immune function can lead to various secondary oral complications, such as candidiasis, herpes simplex virus infection, and periodontal bone loss.
Dental Erosion
GASTROESOPHAGEAL REFLUX DISEASE
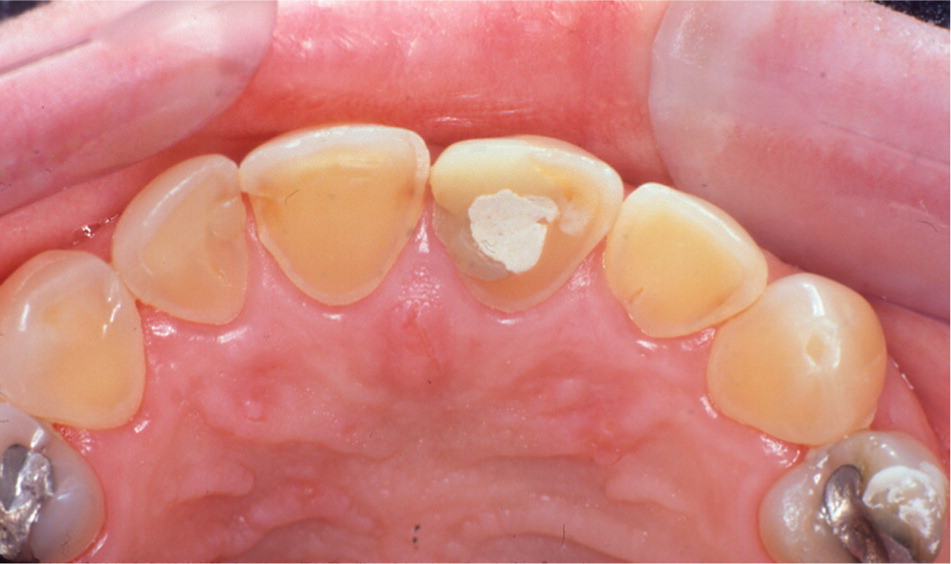
Potential oral findings in patients with gastroesophageal reflux disease include water brash (periodic increase in salivation), xerostomia (dry mouth), burning sensation, halitosis, palatal erythema, and dental erosion. The erosion pattern in patients with gastroesophageal reflex disease tends to favor the occlusal surfaces of the mandibular posterior teeth and the lingual surfaces of the maxillary anterior teeth (Figure 11). Affected teeth exhibit worn, shiny enamel; they may appear yellow and become sensitive to temperature changes as the underlying dentin becomes exposed. Dental erosion is irreversible and may require dental restorative treatment depending on severity; other oral findings usually will resolve with medical management of gastroesophageal reflex disease.
BULIMIA AND ANOREXIA
Potential oral or head and neck findings of bulimia and anorexia include dental erosion, xerostomia, increased rate of caries, and sialadenosis. Vomiting exposes teeth to acidic gastric contents, which leads to enamel erosion. The erosion pattern tends to involve the lingual surfaces of the maxillary anterior teeth (Figure 12) and, in severe cases, the buccal surfaces of the posterior mandibular teeth.39 Patients may have dental sensitivity to cold or sweet stimuli. Xerostomia may be caused by medications often used by patients with bulimia or anorexia (e.g., antidepressants, diuretics, laxatives), as well as by vomiting and excessive exercise.40 Because the buffering and cleansing properties of saliva are important for prevention of tooth decay, xerostomia leads to increased caries risk. Additionally, sialadenosis affects approximately 25 percent of patients with bulimia; bilateral parotid enlargement is the most common presentation.41
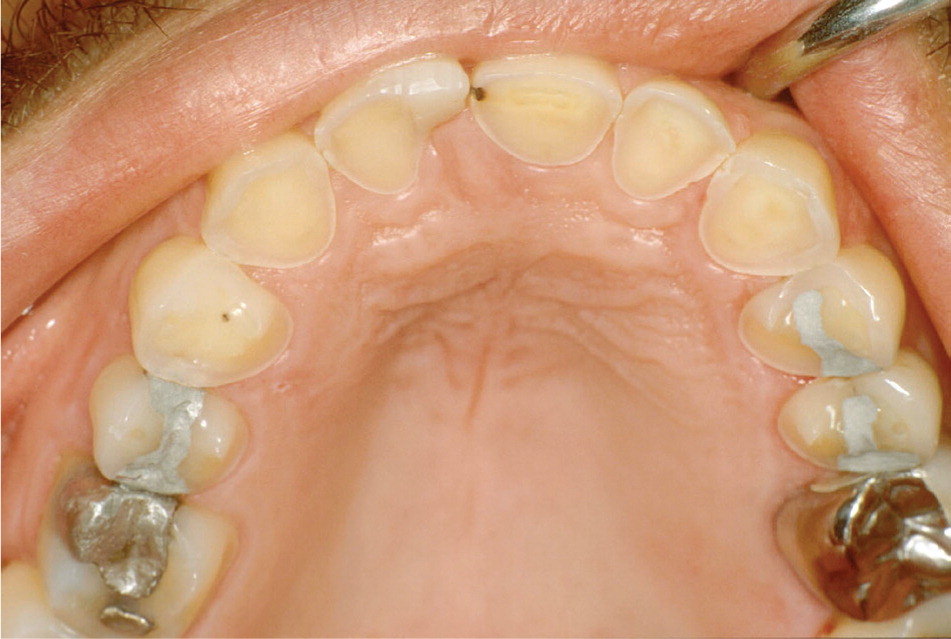
Dental erosion is irreversible and may be addressed by dental restorative procedures. Xerostomia and sialadenosis usually resolve after normalization of nutritional status, although sialagogues may also be helpful.42
Figures 5, 6, and 12 provided by Michele C. Ravenel, DMD.
Figure 10 provided by Michael W. Tabor, DDS.
Figure 11 provided by John McDowell, DDS, MS.
