
Am Fam Physician. 2010;82(12):1499-1506
Patient information: See related handout on side effects of hormonal contraceptives, written by the author of this article.
Author disclosure: Nothing to disclose.
Adverse effects of hormonal contraceptives usually diminish with continued use of the same method. Often, physicians only need to reassure patients that these symptoms will likely resolve within three to five months. Long-acting injectable depot medroxyprogesterone acetate is the only hormonal contraceptive that is consistently associated with weight gain; other hormonal methods are unlikely to increase weight independent of lifestyle choices. Switching combined oral contraceptives is not effective in treating headaches, nor is the use of multivitamins or diuretics. There are no significant differences among various combined oral contraceptives in terms of breast tenderness, mood changes, and nausea. Breakthrough bleeding is common in the first months of combined oral contraceptive use. If significant abnormal bleeding persists beyond three months, other methods can be considered, and the patient may need to be evaluated for other causes. Studies of adverse sexual effects in women using hormonal contraceptives are inconsistent, and the pharmacologic basis for these symptoms is unclear. If acne develops or worsens with progestin-only contraceptives, the patient should be switched to a combination method if she is medically eligible. There is insufficient evidence of any effect of hormonal contraceptives on breast milk quantity and quality. Patient education should be encouraged to decrease the chance of unanticipated adverse effects. Women can also be assessed for medical eligibility before and during the use of hormonal contraceptives.
Adverse effects of hormonal contraceptives usually diminish to the point of acceptance with continued use of the same method. Reassurance that symptoms will likely resolve within three to five months is often the only treatment required. Despite the transient course of these effects, a population-based survey found that 64.6 percent of women who discontinued oral contraceptives did so because of adverse effects.1 Educating patients about common adverse effects of hormonal contraceptives helps to establish realistic expectations (Table 1).1–22
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Physicians should not recommend any combination oral contraceptive over another for decreasing weight gain, headache, breast tenderness, breakthrough bleeding, mood disturbances, acne, or nausea. There are no significant differences among formulations. | C | 9 | Large population-based cohort |
| Physicians should not prescribe progestin-only contraceptives for women who are concerned about breakthrough bleeding. | B | 9 | Large population-based cohort |
| Breakthrough bleeding associated with continuous contraceptive methods can be alleviated by a three- or four-day hormone-free interval. | B | 27, 37 | Randomized controlled trial37 |
| Nonsteroidal anti-inflammatory drugs and estrogens have only short-term benefits in alleviating heavy menses in women who use progestin-only contraceptives. | C | 39, 40 | Evidence-based guideline |
| Patients who have a history of nausea and vomiting with the use of emergency contraceptives should be pretreated with antiemetics. | C | 40 | Evidence-based guideline |
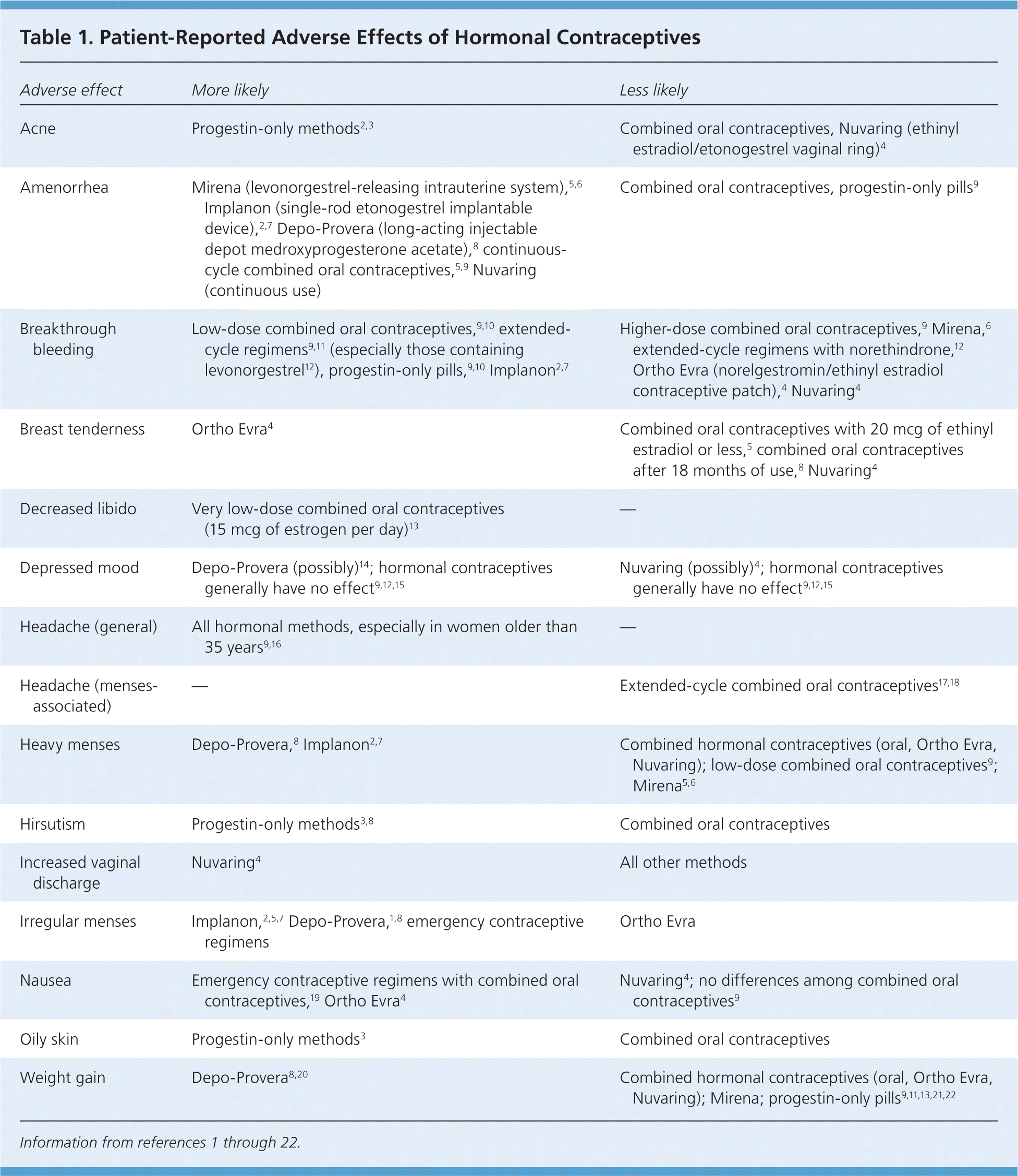
| Adverse effect | More likely | Less likely |
|---|---|---|
| Acne | Progestin-only methods2,3 | Combined oral contraceptives, Nuvaring (ethinyl estradiol/etonogestrel vaginal ring)4 |
| Amenorrhea | Mirena (levonorgestrel-releasing intrauterine system),5,6 Implanon (single-rod etonogestrel implantable device),2,7 Depo-Provera (long-acting injectable depot medroxyprogesterone acetate),8 continuous-cycle combined oral contraceptives,5,9 Nuvaring (continuous use) | Combined oral contraceptives, progestin-only pills9 |
| Breakthrough bleeding | Low-dose combined oral contraceptives,9,10 extended-cycle regimens9,11 (especially those containing levonorgestrel12), progestin-only pills,9,10 Implanon2,7 | Higher-dose combined oral contraceptives,9 Mirena,6 extended-cycle regimens with norethindrone,12 Ortho Evra (norelgestromin/ethinyl estradiol contraceptive patch),4 Nuvaring4 |
| Breast tenderness | Ortho Evra4 | Combined oral contraceptives with 20 mcg of ethinyl estradiol or less,5 combined oral contraceptives after 18 months of use,8 Nuvaring4 |
| Decreased libido | Very low-dose combined oral contraceptives (15 mcg of estrogen per day)13 | — |
| Depressed mood | Depo-Provera (possibly)14; hormonal contraceptives generally have no effect9,12,15 | Nuvaring (possibly)4; hormonal contraceptives generally have no effect9,12,15 |
| Headache (general) | All hormonal methods, especially in women older than 35 years9,16 | — |
| Headache (menses-associated) | — | Extended-cycle combined oral contraceptives17,18 |
| Heavy menses | Depo-Provera,8 Implanon2,7 | Combined hormonal contraceptives (oral, Ortho Evra, Nuvaring); low-dose combined oral contraceptives9; Mirena5,6 |
| Hirsutism | Progestin-only methods3,8 | Combined oral contraceptives |
| Increased vaginal discharge | Nuvaring4 | All other methods |
| Irregular menses | Implanon,2,5,7 Depo-Provera,1,8 emergency contraceptive regimens | Ortho Evra |
| Nausea | Emergency contraceptive regimens with combined oral contraceptives,19 Ortho Evra4 | Nuvaring4; no differences among combined oral contraceptives9 |
| Oily skin | Progestin-only methods3 | Combined oral contraceptives |
| Weight gain | Depo-Provera8,20 | Combined hormonal contraceptives (oral, Ortho Evra, Nuvaring); Mirena; progestin-only pills9,11,13,21,22 |
Persistent symptoms are often alleviated by changing methods; however, no method has been proven superior in terms of adverse effects.9 Exogenous estrogen increases production of sex hormone–binding globulin.23 Progestins bind sex hormone–binding globulin and decrease its synthesis to varying degrees, which should result in different levels of androgenicity. However, despite their biochemical differences, progestins have shown few differences clinically.5
Physicians can decrease the likelihood of significant adverse effects by using criteria from the World Health Organization to assess patients for medical eligibility before and during the use of hormonal contraceptives (Table 2).24,25 Table 3 outlines potential treatments for adverse effects of hormonal contraceptives.1,4,7–13,16,19,22,23,25–34
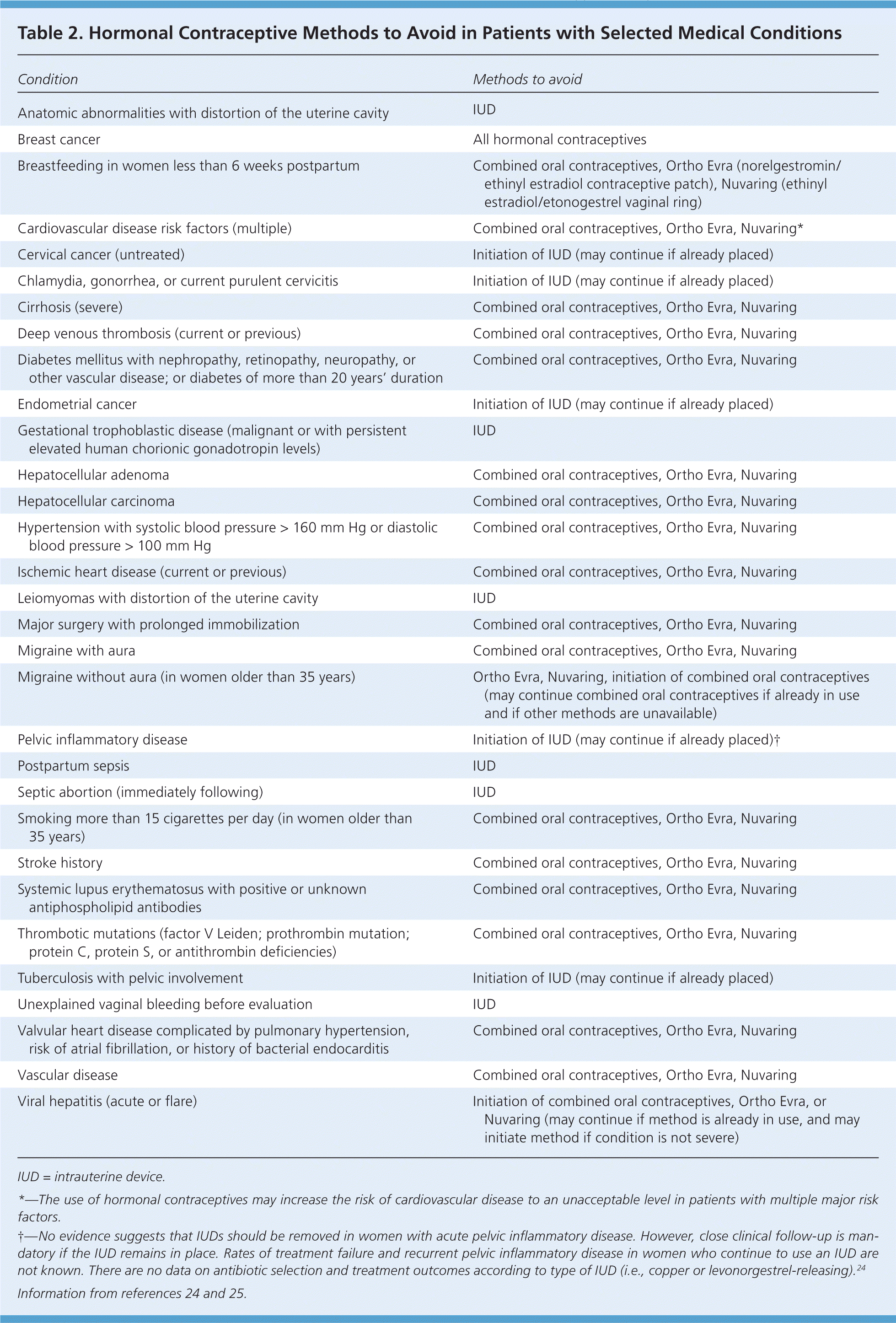
| Condition | Methods to avoid |
|---|---|
| Anatomic abnormalities with distortion of the uterine cavity | IUD |
| Breast cancer | All hormonal contraceptives |
| Breastfeeding in women less than 6 weeks postpartum | Combined oral contraceptives, Ortho Evra (norelgestromin/ethinyl estradiol contraceptive patch), Nuvaring (ethinyl estradiol/etonogestrel vaginal ring) |
| Cardiovascular disease risk factors (multiple) | Combined oral contraceptives, Ortho Evra, Nuvaring* |
| Cervical cancer (untreated) | Initiation of IUD (may continue if already placed) |
| Chlamydia, gonorrhea, or current purulent cervicitis | Initiation of IUD (may continue if already placed) |
| Cirrhosis (severe) | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Deep venous thrombosis (current or previous) | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Diabetes mellitus with nephropathy, retinopathy, neuropathy, or other vascular disease; or diabetes of more than 20 years' duration | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Endometrial cancer | Initiation of IUD (may continue if already placed) |
| Gestational trophoblastic disease (malignant or with persistent elevated human chorionic gonadotropin levels) | IUD |
| Hepatocellular adenoma | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Hepatocellular carcinoma | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Hypertension with systolic blood pressure > 160 mm Hg or diastolic blood pressure > 100 mm Hg | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Ischemic heart disease (current or previous) | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Leiomyomas with distortion of the uterine cavity | IUD |
| Major surgery with prolonged immobilization | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Migraine with aura | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Migraine without aura (in women older than 35 years) | Ortho Evra, Nuvaring, initiation of combined oral contraceptives (may continue combined oral contraceptives if already in use and if other methods are unavailable) |
| Pelvic inflammatory disease | Initiation of IUD (may continue if already placed)† |
| Postpartum sepsis | IUD |
| Septic abortion (immediately following) | IUD |
| Smoking more than 15 cigarettes per day (in women older than 35 years) | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Stroke history | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Systemic lupus erythematosus with positive or unknown antiphospholipid antibodies | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Thrombotic mutations (factor V Leiden; prothrombin mutation; protein C, protein S, or antithrombin deficiencies) | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Tuberculosis with pelvic involvement | Initiation of IUD (may continue if already placed) |
| Unexplained vaginal bleeding before evaluation | IUD |
| Valvular heart disease complicated by pulmonary hypertension, risk of atrial fibrillation, or history of bacterial endocarditis | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Vascular disease | Combined oral contraceptives, Ortho Evra, Nuvaring |
| Viral hepatitis (acute or flare) | Initiation of combined oral contraceptives, Ortho Evra, or Nuvaring (may continue if method is already in use, and may initiate method if condition is not severe) |
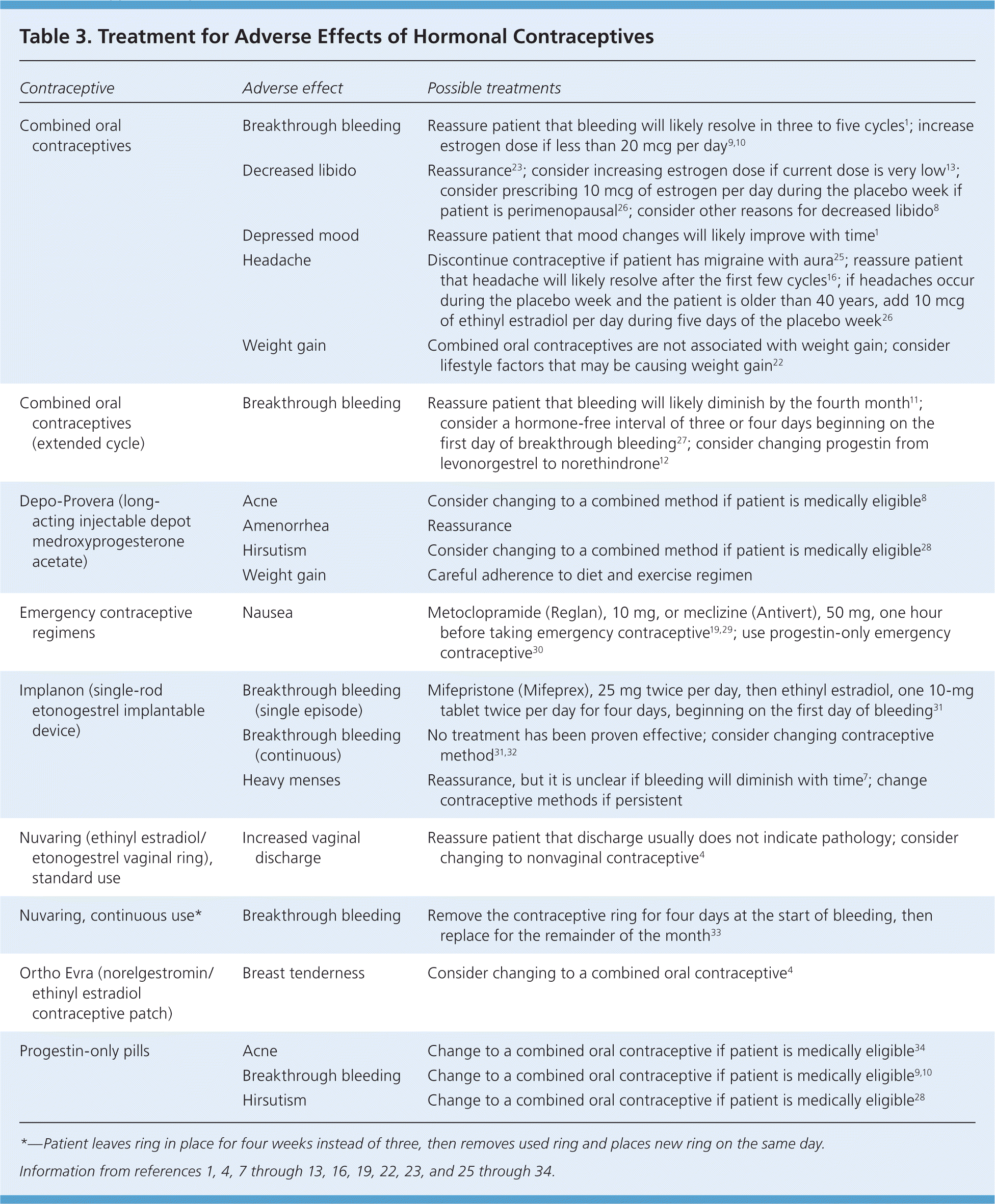
| Contraceptive | Adverse effect | Possible treatments |
|---|---|---|
| Combined oral contraceptives | Breakthrough bleeding | Reassure patient that bleeding will likely resolve in three to five cycles1; increase estrogen dose if less than 20 mcg per day9,10 |
| Decreased libido | Reassurance23; consider increasing estrogen dose if current dose is very low13; consider prescribing 10 mcg of estrogen per day during the placebo week if patient is perimenopausal26; consider other reasons for decreased libido8 | |
| Depressed mood | Reassure patient that mood changes will likely improve with time1 | |
| Headache | Discontinue contraceptive if patient has migraine with aura25; reassure patient that headache will likely resolve after the first few cycles16; if headaches occur during the placebo week and the patient is older than 40 years, add 10 mcg of ethinyl estradiol per day during five days of the placebo week26 | |
| Weight gain | Combined oral contraceptives are not associated with weight gain; consider lifestyle factors that may be causing weight gain22 | |
| Combined oral contraceptives (extended cycle) | Breakthrough bleeding | Reassure patient that bleeding will likely diminish by the fourth month11; consider a hormone-free interval of three or four days beginning on the first day of breakthrough bleeding27; consider changing progestin from levonorgestrel to norethindrone12 |
| Depo-Provera (long-acting injectable depot medroxyprogesterone acetate) | Acne | Consider changing to a combined method if patient is medically eligible8 |
| Amenorrhea | Reassurance | |
| Hirsutism | Consider changing to a combined method if patient is medically eligible28 | |
| Weight gain | Careful adherence to diet and exercise regimen | |
| Emergency contraceptive regimens | Nausea | Metoclopramide (Reglan), 10 mg, or meclizine (Antivert), 50 mg, one hour before taking emergency contraceptive19,29; use progestin-only emergency contraceptive30 |
| Implanon (single-rod etonogestrel implantable device) | Breakthrough bleeding (single episode) | Mifepristone (Mifeprex), 25 mg twice per day, then ethinyl estradiol, one 10-mg tablet twice per day for four days, beginning on the first day of bleeding31 |
| Breakthrough bleeding (continuous) | No treatment has been proven effective; consider changing contraceptive method31,32 | |
| Heavy menses | Reassurance, but it is unclear if bleeding will diminish with time7; change contraceptive methods if persistent | |
| Nuvaring (ethinyl estradiol/etonogestrel vaginal ring), standard use | Increased vaginal discharge | Reassure patient that discharge usually does not indicate pathology; consider changing to nonvaginal contraceptive4 |
| Nuvaring, continuous use* | Breakthrough bleeding | Remove the contraceptive ring for four days at the start of bleeding, then replace for the remainder of the month33 |
| Ortho Evra (norelgestromin/ethinyl estradiol contraceptive patch) | Breast tenderness | Consider changing to a combined oral contraceptive4 |
| Progestin-only pills | Acne | Change to a combined oral contraceptive if patient is medically eligible34 |
| Breakthrough bleeding | Change to a combined oral contraceptive if patient is medically eligible9,10 | |
| Hirsutism | Change to a combined oral contraceptive if patient is medically eligible28 |
Weight Gain
Long-acting injectable depot medroxyprogesterone acetate (Depo-Provera) is the only hormonal contraceptive that is consistently associated with weight gain. A prospective study found that women who used Depo-Provera gained an average of 11.2 lb (5.1 kg) over 36 months, whereas women who used combined oral contraceptives did not gain any weight.8,20
There are no significant differences among combined oral contraceptives in terms of weight gain.9 A systematic review of randomized controlled trials did not find a causal connection between combined hormonal contraceptives and weight gain,21 whereas a Cochrane review found the evidence to be insufficient.22 Extended-cycle combined oral contraceptives do not cause more weight gain than standard regimens.11 A randomized prospective trial of two combined oral contraceptive regimens and the ethinyl estradiol/etonogestrel vaginal ring (Nuvaring) did not find significant weight gain in any group.13
Headache
Combined oral contraceptives increase the risk of stroke in women who have migraines with aura, and should not be used in these patients.25 A systematic review found that 10 percent of women have new-onset headache with the use of combined oral contraceptives.16 The type and dose of progestin do not affect headache,16 nor does the particular formulation.9
Headaches are more common during the first cycle of combined oral contraceptives and in women who are older than 35 years.16 If headache occurs in a woman who is older than 40 years during the placebo week of a 28-day regimen, the addition of 10 mcg of ethinyl estradiol for five of the seven placebo days may help.26 It is not known if this regimen is effective in younger women. Continuous use of combined oral contraceptives also can be attempted. A Cochrane review comparing extended-cycle with standard 28-day regimens found slightly reduced rates of menses-associated headache in the extended-cycle group.17 A prospective, open-label, industry-sponsored trial found that switching from a 28-day to a 168-day regimen reduced headache and increased quality-of-life measures in patients with severe headaches, but not in those with mild headaches.18 Switching to a different combined oral contraceptive or taking diuretics or multivitamin supplements is not effective in treating headaches.16
Breast Tenderness
The use of combined oral contraceptives decreases breast tenderness after 18 months, but there are no significant differences among formulations.8,9 Breast tenderness is more common in women who use the norelgestromin/ethinyl estradiol contraceptive patch (Ortho Evra) than in those who use combined oral contraceptives.4
Breakthrough Bleeding
Breakthrough bleeding is common in the first months of combined oral contraceptive use,1 and patients should be reassured during this time. Variations in the estrogen dose above 20 mcg do not alter bleeding rates,9 nor does changing the type of progestin.35 Bleeding patterns are similar among monophasic and biphasic regimens,33 but the evidence is insufficient to determine whether monophasic and triphasic regimens result in different bleeding patterns. 36 A randomized controlled trial comparing continuous use with a standard 28-day cycle found that spotting increased initially with continuous use, but was less than with the standard regimen by nine months.11 Increasing the estrogen dosage from 20 to 30 mcg per day does not reduce breakthrough bleeding in extended-cycle regimens. 12 Women on regimens containing norethindrone had significantly more days of amenorrhea than those on levonorgestrel-containing regimens.12 If breakthrough bleeding occurs with extended-cycle regimens, the pills should be stopped for three or four days, then restarted.27
A prospective randomized trial found that women who have breakthrough bleeding for at least five days with continuous Nuvaring use could reduce bleeding by removing the ring at the start of bleeding, storing it for four days, then replacing the same ring.37
Other Bleeding Irregularities
Patients often discontinue hormonal contraceptives because of menstrual cycle disorders.1 Progestin-only pills and low-dose combined oral contraceptives (less than 20 mcg per day) are associated with a higher incidence of bleeding disturbances.9,10 Compared with nonhormonal contraceptive methods, Depo-Provera is strongly associated with missed menstrual periods and bleeding for longer than 20 days.8 A Cochrane review found that no interventions to regulate menstrual bleeding in women using Depo-Provera were useful in the long term.38 In women using progestin-only injectable contraceptives, short-term treatment with nonsteroidal anti-inflammatory drugs may be helpful for spotting.39 Nonsteroidal anti-inflammatory drugs or ethinyl estradiol can also be used for heavy or prolonged bleeding until another contraceptive method is chosen.39,40
Women who use the single-rod etonogestrel implantable device (Implanon) should expect changes in their menstrual cycle.7 Before insertion, physicians should inform patients that only about 11 percent of women have a normal bleeding pattern.2 A combination of mifepristone (Mifeprex) and ethinyl estradiol reduces the duration of a single bleeding episode in women who use Implanon, but does not alter the overall bleeding pattern. 31,32 One study showed that doxycycline shortens a single episode of bleeding in women who use Implanon, 32 but a subsequent larger study did not confirm this finding.31 If abnormal bleeding persists beyond three months, an alternative contraceptive method may be considered, and the patient may need to be evaluated for other causes.40
Mood
No significant differences in effect on mood have been found among various combined oral contraceptives.9,15 A prospective population-based study found that Depo- Provera was associated with a slightly increased rate of depression, which can persist after discontinuation of therapy.14 A prospective cohort study, however, found that neither combined oral contraceptives nor Depo- Provera was associated with an increased risk of depressive symptoms.8,15
Sexual Effects
Findings from studies of the sexual effects of hormonal contraceptives have been inconsistent, and the pharmacologic basis for these effects is unclear.23 Bioavailable testosterone is lower in women who use combined oral contraceptives than in nonusers; however, one review found that women who use these contraceptives show more interest in erotic images.23 Supplementation with androstenedione (illegal in the United States) or testosterone in combined oral contraceptive users with decreased libido is no more effective than placebo.23
A prospective analysis of women using Depo-Provera found no change in sexual function after four months, and women using progestin-only pills had no difference in sexual desire compared with those receiving placebo.23 Another study found that there was no significant change in libido after 24 months among women using Depo- Provera, women taking an oral contraceptive containing 0.15 mg of desogestrel and 20 mcg of ethinyl estradiol, and women using nonhormonal contraception.8 A prospective randomized study comparing Nuvaring with two combined oral contraceptives (one containing 20 mcg of ethinyl estradiol and 100 mcg of levonorgestrel, and the other containing 15 mcg of ethinyl estradiol and 60 mcg of gestodene) found that the latter pill had the greatest negative effect on sexual desire.13 The reason is not known.
If adverse sexual effects persist beyond three months, the method can be changed, but there is little evidence to recommend one method over another. Because sexual function depends on many factors, other causes of sexual dysfunction should be considered before changing methods.8
Skin Changes
Acne can develop or worsen with the use of progestinonly contraceptives. A questionnaire-based study of 161 women who used the levonorgestrel-releasing intrauterine system (Mirena) for dysfunctional uterine bleeding found that 22 percent discontinued use secondary to progestin-associated effects (e.g., acne, oily skin, hirsutism, bloating, headaches, weight gain, depression, breast tenderness, decreased libido).3 A retrospective study of Implanon users found that 11 percent had acne after insertion.2 If acne worsens with a progestin-only contraceptive, a combination method can be tried if the patient is medically eligible.
Of patients who had acne at baseline and who were using a combined oral contraceptive with 20 mcg of ethinyl estradiol and 0.15 mg of desogestrel, 70 percent had resolution of symptoms at six months.8 If acne does not improve within six months of combined oral contraceptive use, it typically will not improve with continued use.8 A Cochrane review analyzing several combined oral contraceptive regimens found that they are effective in treating acne, but that differences in effectiveness among progestins is not clear.34 A separate Cochrane review found that Nuvaring users reported less acne than women taking combined oral contraceptives.4
Nausea
Levonorgestrel-only emergency contraceptives cause less nausea and vomiting than regimens with a combination of ethinyl estradiol and levonorgestrel.20 Pretreatment with metoclopramide (Reglan) or meclizine (Antivert) can reduce nausea in women using combined oral contraceptives for emergency contraception.29,30 The World Health Organization advises pretreatment for women who have a history of nausea and vomiting with emergency contraceptive use.40 For nonemergency use, there are no significant differences in nausea among combined oral contraceptive formulations.9,12
Decreased Breast Milk
A Cochrane review found insufficient evidence that hormonal contraceptives affects breast milk quantity or quality.42 Combined oral contraceptives should not be used for the first six weeks postpartum because of increased risk of hypercoagulability.25,43 Depo-Provera and progestin-only pills do not impair lactation.43,44
Patient Education
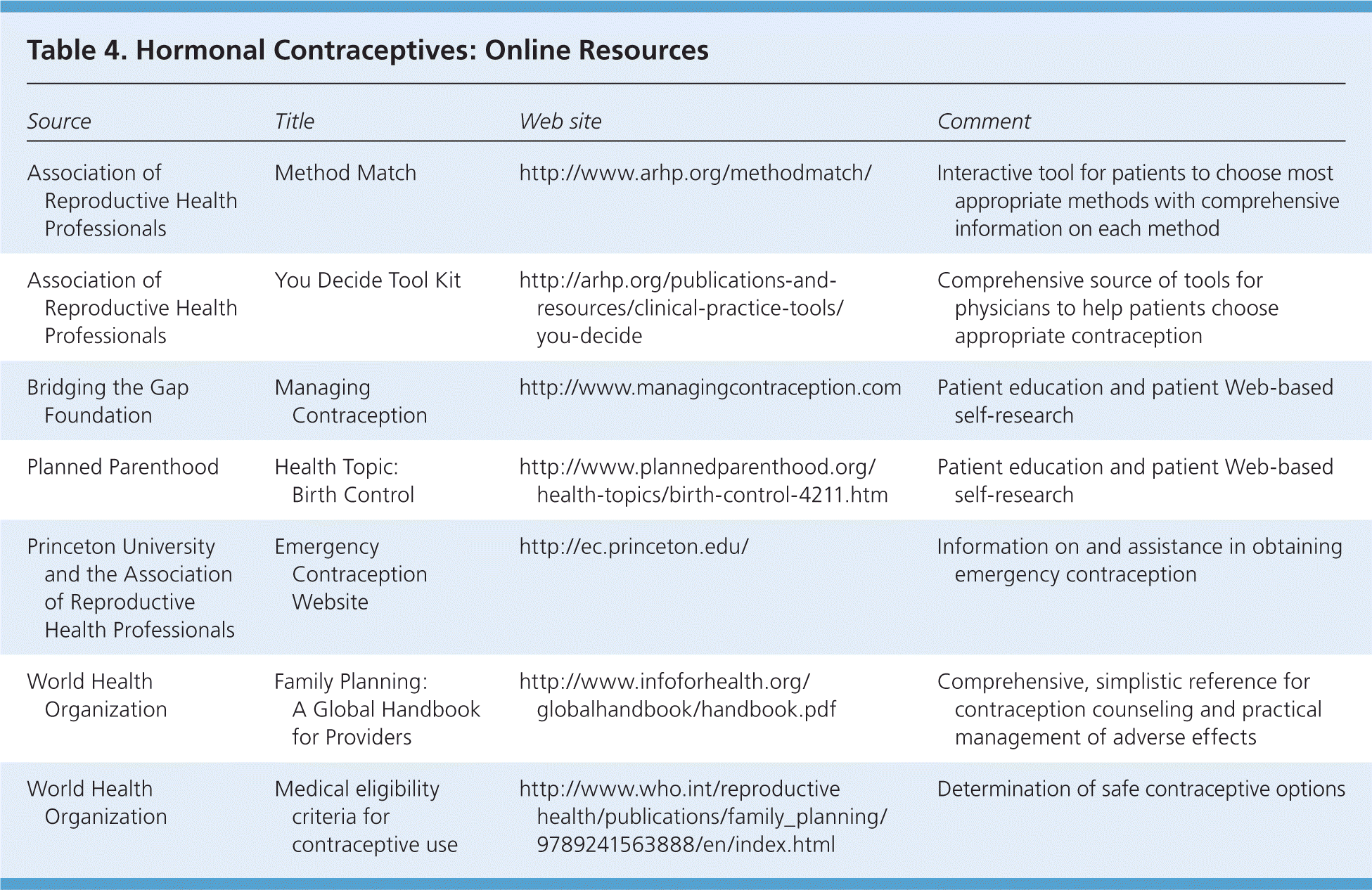
Patient education can decrease the chances of unanticipated adverse effects of hormonal contraceptives. Table 4 lists resources for physicians and patients.
| Source | Title | Web site | Comment |
|---|---|---|---|
| Association of Reproductive Health Professionals | Method Match | http://www.arhp.org/methodmatch/ | Interactive tool for patients to choose most appropriate methods with comprehensive information on each method |
| Association of Reproductive Health Professionals | You Decide Tool Kit | http://arhp.org/publications-and-resources/clinical-practice-tools/you-decide | Comprehensive source of tools for physicians to help patients choose appropriate contraception |
| Bridging the Gap Foundation | Managing Contraception | http://www.managingcontraception.com | Patient education and patient Web-based self-research |
| Planned Parenthood | Health Topic: Birth Control | http://www.plannedparenthood.org/health-topics/birth-control-4211.htm | Patient education and patient Web-based self-research |
| Princeton University and the Association of Reproductive Health Professionals | Emergency Contraception Website | http://ec.princeton.edu/ | Information on and assistance in obtaining emergency contraception |
| World Health Organization | Family Planning: A Global Handbook for Providers | http://www.infoforhealth.org/globalhandbook/handbook.pdf | Comprehensive, simplistic reference for contraception counseling and practical management of adverse effects |
| World Health Organization | Medical eligibility criteria for contraceptive use | http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/index.html | Determination of safe contraceptive options |
