
Am Fam Physician. 2010;82(12):1512-1514
End-stage renal disease (ESRD) affects more than 1,500 persons per 1 million population in countries with a high prevalence, such as the United States and Japan. About two-thirds of persons with ESRD receive hemodialysis, one-fourth have kidney transplants, and one-tenth receive peritoneal dialysis.
Risk factors for ESRD include older age; hypertension; diabetes mellitus; obesity; history of renal disease; and tobacco, heroin, or analgesic use.
ESRD leads to fluid retention, anemia, disturbances of bone and mineral metabolism, and increased risk of cardiovascular disease (CVD).
Increasing the dose of peritoneal dialysis does not seem to reduce mortality.
In persons receiving hemodialysis, there is no difference in mortality for high flux hemodialysis compared with low flux hemodialysis, or increased-dose compared with standard-dose hemodialysis.
Erythropoietin and darbepoetin may help maintain hemoglobin levels in persons with ESRD, although normalizing hemoglobin levels in persons with both ESRD and CVD may increase mortality.
Disorders of calcium and phosphate metabolism may contribute to the increased risk of CVD in persons with ESRD.
Phosphate binders (sevelamer) may slow arterial calcification and reduce serum low-density lipoprotein cholesterol levels, but we do not know whether this reduces cardiovascular events or mortality.
Cinacalcet is more effective than placebo at improving control of secondary hyperparathyroidism, but we do not know whether it reduces cardiovascular events or mortality.
Mupirocin reduces Staphylococcus aureus infections compared with placebo or no treatment.
The use of statins in persons with ESRD does not seem to reduce mortality or cardiovascular events.
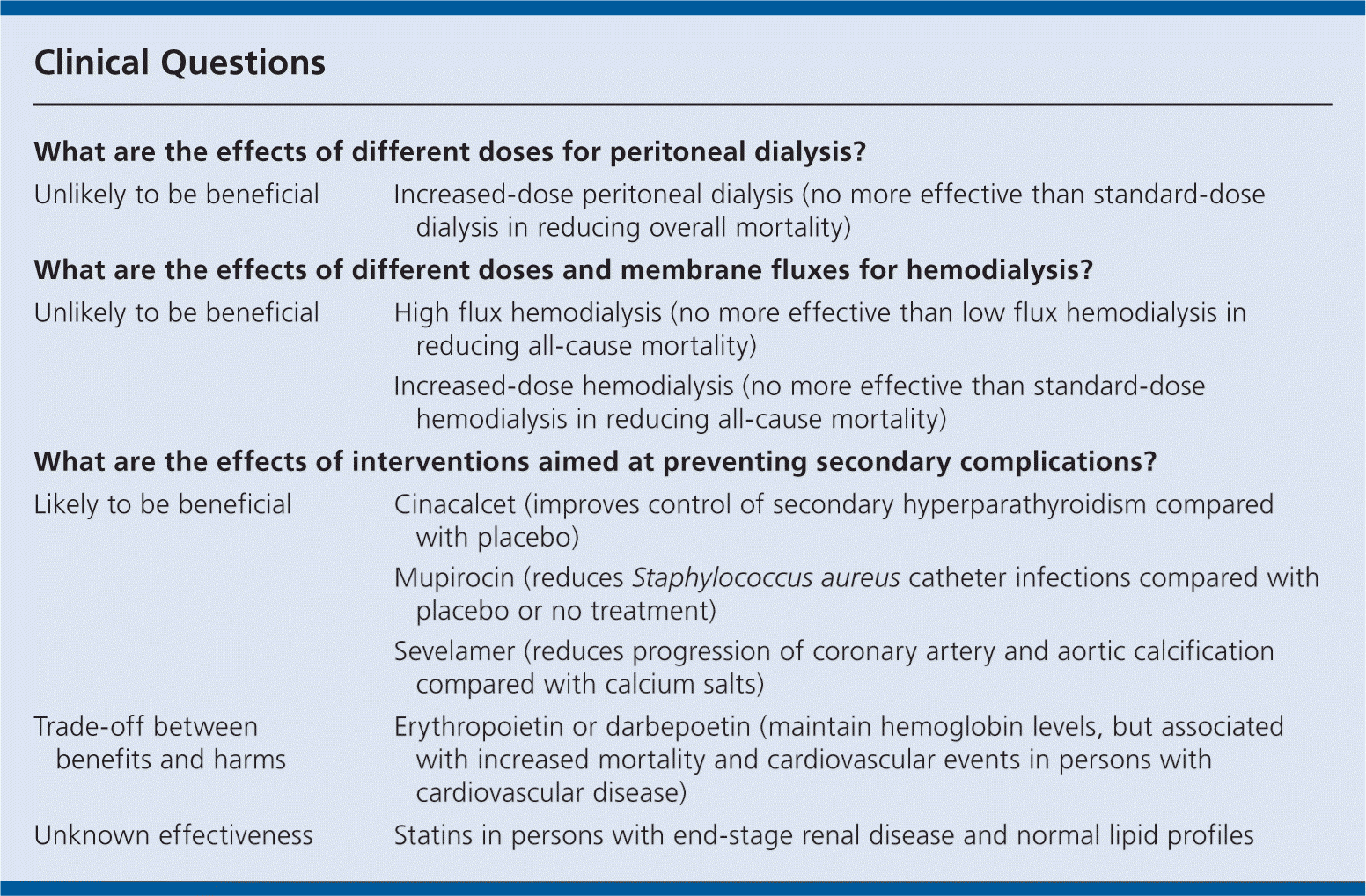
| What are the effects of different doses for peritoneal dialysis? | |
| Unlikely to be beneficial | Increased-dose peritoneal dialysis (no more effective than standard-dose dialysis in reducing overall mortality) |
| What are the effects of different doses and membrane fluxes for hemodialysis? | |
| Unlikely to be beneficial | High flux hemodialysis (no more effective than low flux hemodialysis in reducing all-cause mortality) |
| Increased-dose hemodialysis (no more effective than standard-dose hemodialysis in reducing all-cause mortality) | |
| What are the effects of interventions aimed at preventing secondary complications? | |
| Likely to be beneficial | Cinacalcet (improves control of secondary hyperparathyroidism compared with placebo) Mupirocin (reduces Staphylococcus aureus catheter infections compared with placebo or no treatment) |
| Sevelamer (reduces progression of coronary artery and aortic calcification compared with calcium salts) | |
| Trade-off between benefits and harms | Erythropoietin or darbepoetin (maintain hemoglobin levels, but associated with increased mortality and cardiovascular events in persons with cardiovascular disease) |
| Unknown effectiveness | Statins in persons with end-stage renal disease and normal lipid profiles |
Definition
ESRD is defined as irreversible decline in kidney function that is severe enough to be fatal in the absence of dialysis or transplantation. ESRD is included under stage 5 of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative classification of chronic kidney disease, where it refers to persons with an estimated glomerular filtration rate less than 15 mL per minute per 1.73 m2 body surface area, or those requiring dialysis irrespective of glomerular filtration rate. Reduction in or absence of kidney function leads to a host of maladaptive changes, including fluid retention (extracellular volume overload), anemia, disturbances of bone and mineral metabolism, dyslipidemia, and protein-energy malnutrition. This review deals with ESRD in adults only.
Fluid retention in persons with ESRD contributes significantly to the hypertension, ventricular dysfunction, and excess cardiovascular events observed in this population. Anemia associated with chronic kidney disease is normocytic and normochromic, and is most commonly attributed to reduced erythropoietin synthesis by the affected kidneys. Additional factors that contribute to the anemia include iron deficiency from frequent phlebotomy, blood retention in the dialyzer and tubing, and gastrointestinal bleeding; severe secondary hyperparathyroidism; acute and chronic inflammatory conditions (e.g., infection); and shortened red blood cell survival. Disturbances of bone and mineral metabolism, such as hyperparathyroidism, hyperphosphatemia, and hypo- or hypercalcemia, are common in persons with chronic kidney disease. If untreated, these disturbances can cause pain, pruritus, anemia, bone loss, and increased fracture risk, and can contribute to hypertension and CVD.
Incidence and Prevalence
According to the U.S. Renal Data System 2009 annual report, there were 111,000 new cases of ESRD in 2007—equivalent to an annual incidence of 361 cases per 1 million population. The prevalence of ESRD in the United States in 2007 was 527,283 (1,698 cases per 1 million population). According to international comparative data published in the U.S. Renal Data System 2009 annual report, the highest incidence (415 per 1 million population) and prevalence (2,288 cases per 1 million population) of ESRD in 2007 worldwide occurred in Taiwan. In 2007, Japan also observed a relatively high incidence (285 per 1 million population) and prevalence (2,060 cases per 1 million population) of ESRD, which included only persons receiving maintenance dialysis.
In comparison, the incidence of treated ESRD among all registries reporting to the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry was 116 per 1 million population in 2007. The overall prevalence of treated ESRD in 2007 among all registries reporting to the ERA-EDTA Registry was 662 per 1 million population. In 2007, the Australia and New Zealand Dialysis and Transplant Registry reported an annual incidence of treated ESRD of 110 persons per 1 million population in Australia and 109 persons per 1 million population in New Zealand. The prevalence of treated ESRD in 2007 was 797 persons per 1 million population in Australia and 793 persons per 1 million population in New Zealand.
Etiology
The amount of daily proteinuria remains one of the strongest predictors of progression to ESRD. Hypertension is a strong independent risk factor for progression to ESRD, particularly in persons with proteinuria. Age is also a predictor of ESRD; persons older than 65 years have a four- to fivefold increased risk of ESRD compared with persons younger than 65 years. Additional risk factors for developing ESRD include a history of chronic renal insufficiency, diabetes, heroin abuse, tobacco or analgesic use, non-white race or ethnicity, lower socioeconomic status, obesity, hyperuricemia, and a family history of kidney disease.
Prognosis
The overall prognosis of untreated ESRD is poor. Most persons with ESRD eventually die from complications of CVD, infection, or, if dialysis is not provided, progressive uremia (hyperkalemia, acidosis, malnutrition, altered mental functioning). Precise mortality estimates, however, are unavailable because international renal registries omit persons with ESRD who do not receive renal replacement therapy. Among persons receiving renal replacement therapy, CVD is the leading cause of mortality and accounts for more than 40 percent of deaths in this population. Extracellular volume overload and hypertension—which are common among persons with chronic kidney disease—are known predictors of left ventricular hypertrophy and cardiovascular mortality in this population. Even after adjustment for age, sex, race or ethnicity, and the presence of diabetes, annual cardiovascular mortality remains roughly an order of magnitude higher in persons with ESRD than in the general population, particularly among younger persons.
