
Am Fam Physician. 2010;82(12):1526-1527
Summary of Recommendation and Evidence
The U.S. Preventive Services Task Force (USPSTF) recommends that all women planning or capable of pregnancy take a daily supplement containing 0.4 to 0.8 mg (400 to 800 mcg) of folic acid (Table 1). A recommendation.
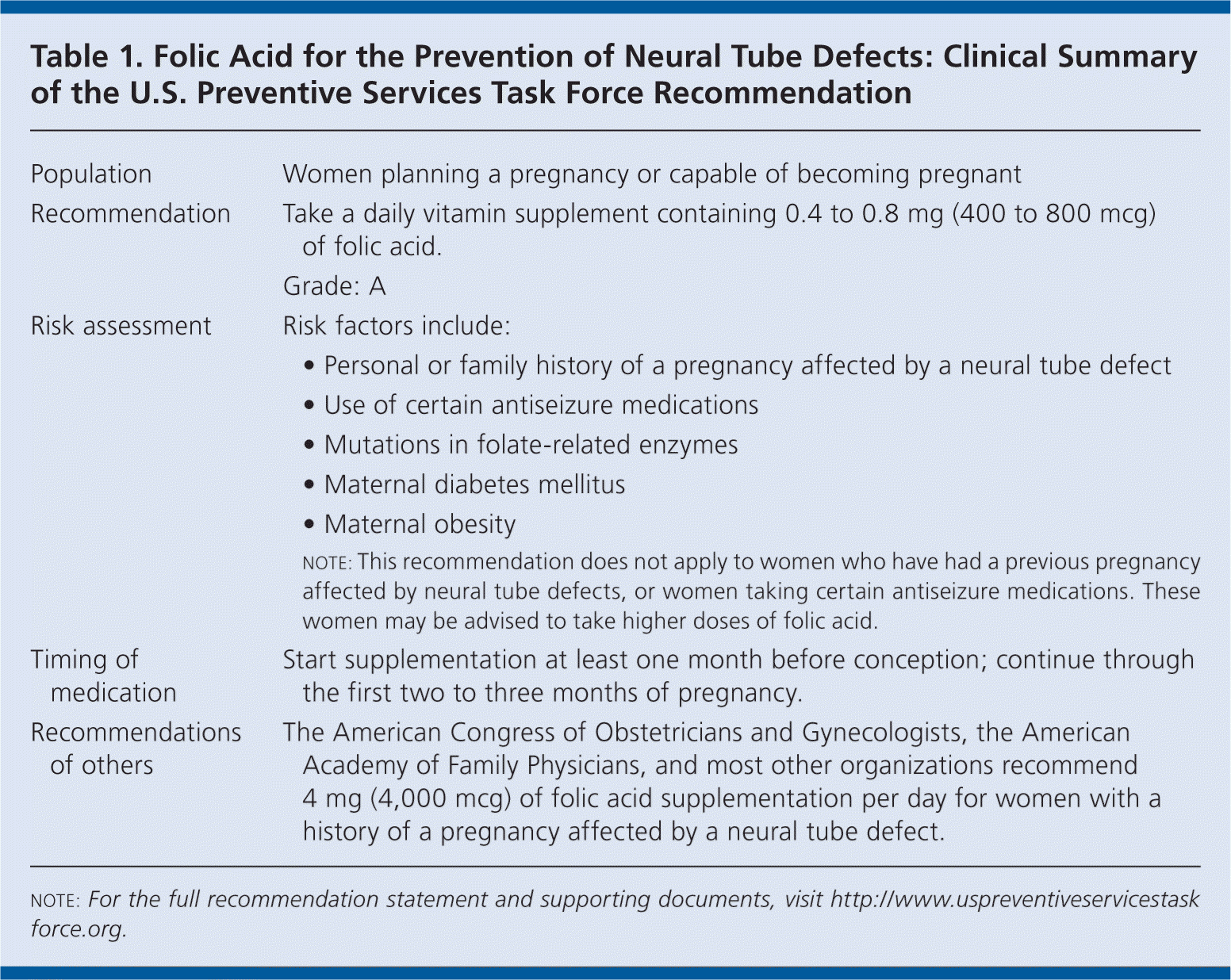
| Population | Women planning a pregnancy or capable of becoming pregnant |
| Recommendation | Take a daily vitamin supplement containing 0.4 to 0.8 mg (400 to 800 mcg) of folic acid. |
| Grade: A | |
| Risk assessment | Risk factors include:
|
| Timing of medication | Start supplementation at least one month before conception; continue through the first two to three months of pregnancy. |
| Recommendations of others | The American Congress of Obstetricians and Gynecologists, the American Academy of Family Physicians, and most other organizations recommend 4 mg (4,000 mcg) of folic acid supplementation per day for women with a history of a pregnancy affected by a neural tube defect. |
Rationale
Importance. Approximately one in every 1,000 pregnancies is affected by a neural tube defect.
Recognition of risk status. Although a personal or family history of a pregnancy affected by a neural tube defect is associated with an increased risk of having an affected pregnancy, most cases occur in the absence of any positive history.
Benefits of preventive medication. The USPSTF found convincing evidence that supplements containing 0.4 to 0.8 mg (400 to 800 mcg) of folic acid in the periconceptional period reduce the risk of neural tube defects.
Harms of preventive medication. Adequate evidence suggests that folic acid from supplementation at usual doses is not associated with serious harms.
USPSTF assessment. The USPSTF concludes that, for women who are planning or capable of pregnancy, there is high certainty that the net benefit is substantial.
Clinical Considerations
Patient population. This recommendation applies to women who are planning or capable of pregnancy, but it does not apply to women who have had a previous pregnancy affected by neural tube defects or women taking certain antiseizure medications. Most organizations recommend that these women take higher doses of folic acid.
Assessment of risk. The use of certain antiseizure medications and a personal or family history of neural tube defects are well-established risk factors. Other reported risk factors include mutations in folate-related enzymes, maternal diabetes mellitus, and obesity.
Timing. Most studies indicate the need to start daily folic acid supplementation at least one month before conception and to continue through the first two to three months of pregnancy. Studies also indicate that 50 percent of pregnancies in the United States are unplanned, and therefore, clinicians should advise all women who are capable of pregnancy to take folic acid supplements.
Dosage. Good evidence from randomized trials in settings without fortification of food suggests that a multivitamin with 0.8 mg (800 mcg) of folic acid reduces the risk of neural tube defects. Observational studies done before fortification report a reduction of neural tube defects in women taking a supplement with 0.4 mg (400 mcg) of folic acid (the generally available dose). Evidence indicates that most women in the United States are not ingesting fortified foods at a level thought to provide optimal benefit. In a setting in which food is fortified with folic acid, the effective amount of additional folic acid supplementation is unclear.
