
Am Fam Physician. 2010;82(12):1553-1554
Author disclosure: Nothing to disclose.
Clinical Question
Is thiazolidinedione (TZD) therapy effective for treating metabolic syndrome?
Evidence-Based Answer
There is no patient-oriented evidence supporting the use of TZD therapy in patients in the general population who have metabolic syndrome. Rosiglitazone (Avandia) use decreases cardiovascular morbidity and mortality in patients with metabolic syndrome who are undergoing coronary stenting. (Strength of Recommendation: B, based on a single randomized controlled trial). Effects of TZD therapy on nonglycemic markers of metabolic syndrome are inconsistent compared with the effects of other hypoglycemic medications.
Evidence Summary
A randomized controlled trial evaluated patient-oriented outcomes of TZD therapy in patients with metabolic syndrome but without diabetes mellitus.1 In 360 patients with metabolic syndrome who were undergoing coronary stent implantation, rosiglitazone use reduced the incidence of major adverse cardiac events (defined as cardiac death or target lesion revascularization) compared with placebo (7.2 versus 14.5 percent; P = .04; number need to treat = 13.7).1 The reduction in major adverse cardiac events was primarily the result of a reduction in target vessel revascularization (4.6 versus 11.7 percent; P = .02). There were no significant differences in the rates of myocardial infarction (2.6 versus 2.8 percent) or heart failure (2.6 versus 1.4 percent) that were attributable to rosiglitazone.
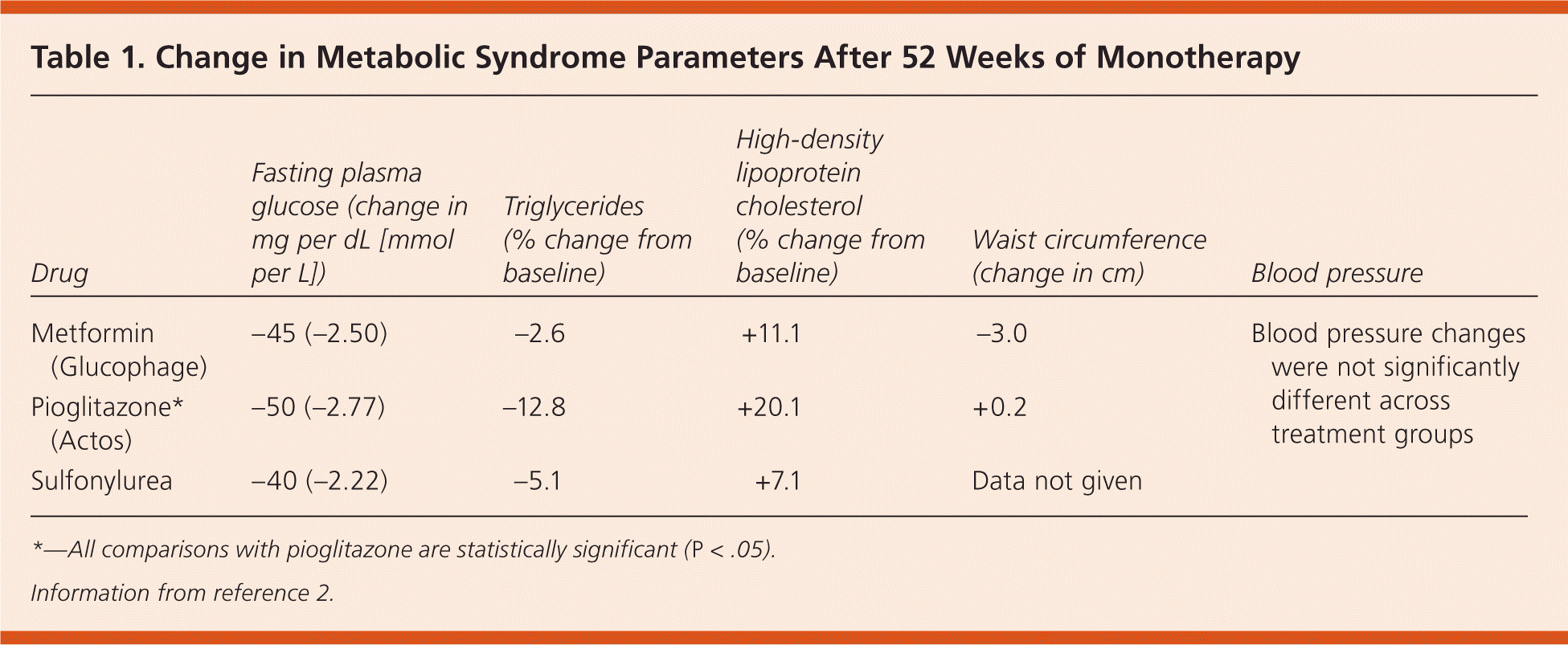
A review of four randomized controlled trials examined 3,186 patients with type 2 diabetes who met the National Cholesterol Education Program, Adult Treatment Panel III (NCEP-ATP III) criteria for metabolic syndrome.2 Treatment was truncated at one year in each trial. Patients receiving pioglitazone (Actos) monotherapy (n = 1,040) had a greater decrease in fasting plasma glucose and triglyceride levels, and a greater increase in high-density lipoprotein levels compared with patients receiving metformin (Glucophage) monotherapy (n = 500) or sulfonylurea monotherapy (n = 535). However, pioglitazone use was associated with an increase in waist circumference, compared with a decrease in patients taking metformin. Blood pressure was not significantly different between treatment groups.2 Table 1 shows the changes in these parameters after one year.2 It should be noted that the authors of this review were employed by the makers of Actos, and the four trials included in the review were also sponsored by the company.
| Drug | Fasting plasma glucose (change in mg per dL [mmol per L]) | Triglycerides (% change from baseline) | High-density lipoprotein cholesterol (% change from baseline) | Waist circumference (change in cm) | Blood pressure |
|---|---|---|---|---|---|
| Metformin (Glucophage) | –45 (–2.50) | –2.6 | +11.1 | –3.0 | Blood pressure changes were not significantly different across treatment groups |
| Pioglitazone* (Actos) | –50 (–2.77) | –12.8 | +20.1 | +0.2 | |
| Sulfonylurea | –40 (–2.22) | –5.1 | +7.1 | Data not given |
A meta-analysis of 87 randomized controlled trials compared the effects of rosiglitazone and pioglitazone in patients with type 2 diabetes, prediabetes, or metabolic syndrome, using three different definitions of metabolic syndrome.3 The limited number and heterogeneity of the studies prevented a separate meta-analysis of patients with metabolic syndrome. There were insufficient data to determine whether the two medications differed in their effects on progression to diabetes. Data from the metaanalysis were also insufficient to compare the effects of pioglitazone and rosiglitazone on cardiovascular morbidity and mortality.
A nonsystematic review of the metabolic effects of TZD therapy concluded that data were insufficient to recommend routine use of TZDs in patients with metabolic syndrome.4
Recommendations from Others
The guidelines from the NCEP-ATP III expert panel, which were updated in 2004, do not make a recommendation for or against TZD therapy in patients with metabolic syndrome.5,6 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends intensive lifestyle modification for persons with metabolic syndrome; medications may be used as appropriate for treating individual components of the syndrome.7 In addition to lifestyle modification, the American Heart Association recommends that a fibrate, such as fenofibrate (Tricor) or gemfibrozil (Lopid), can be combined with a statin in patients with high triglyceride levels who have metabolic syndrome or diabetes.8 However, it does not mention TZD therapy for managing metabolic syndrome.
