
Am Fam Physician. 2011;83(2):121-127
Author disclosure: Nothing to disclose.
In March 2010, the National Institutes of Health (NIH) convened a consensus conference on vaginal birth after previous cesarean delivery (VBAC),1 using a systematic review from the Agency for Healthcare Research and Quality as its primary source of evidence.2 The conference occurred in the setting of an increasing U.S. cesarean delivery rate, which reached an all-time high of 32.3 percent in 2008.3 This increase was driven in part by a sharp decrease in the VBAC rate, from 28.3 percent in 1996 to 8.5 percent in 2006.4 An important factor in the decreased rate of VBAC is the inability of maternity care units to meet recommendations from the American College of Obstetricians and Gynecologists (ACOG), which stipulate that a trial of labor after previous cesarean delivery (TOLAC) should occur only in facilities with “immediately available” surgeons and anesthesiologists.5,6
The increasing rate of cesarean deliveries and the lack of access to TOLAC have created a major public health concern. Maternal morbidity and mortality seem to be increasing in the United States, partly as a result of the increase in repeat cesarean deliveries. The 2006 maternal mortality rate (13.3 deaths per 100,000 births7) is well above the Healthy People 2010 target of 3.3 per 100,000 births.8,9 The maternal mortality rate associated with elective repeat cesarean delivery is 13.4 deaths per 100,000 births, compared with 3.8 per 100,000 births for TOLAC.1
The risk of bladder injury, hysterectomy, and blood transfusion increases with a woman's number of previous cesarean deliveries.2,10 Pregnant women with three prior cesarean deliveries have a 3 percent risk of placenta previa, compared with 0.95 percent in women with a single prior cesarean delivery.1 The potential for neonatal death and neurologic injury resulting from maternal uterine rupture prompted ACOG's “immediately available” requirement. TOLAC is associated with a perinatal mortality rate of 1.3 deaths per 1,000 births, compared with 0.5 per 1,000 births for elective repeat cesarean delivery.2 It is important for physicians and patients to appreciate that the perinatal mortality rate for infants born to women attempting TOLAC is similar to that for all infants born to laboring nulliparous women.
The NIH consensus statement expresses concern about barriers faced by women who desire TOLAC, and recommends that ACOG and the American Society of Anesthesiologists consider the risks of TOLAC in the context of other obstetric risks.1 The newly updated ACOG guideline (summarized in the Practice Guideline in this issue of American Family Physician11) continues to recommend that TOLAC occur only in facilities with personnel immediately available for emergency cesarean delivery.12 Recognizing that this may not be feasible in smaller and rural hospitals, ACOG states that “respect for patient autonomy supports the concept that patients should be allowed to accept increased levels of risk, however, patients should be clearly informed of such potential increase in risk and management alternatives.”12 The guideline also contains several other provisions that may help increase the accessibility of TOLAC, including removing or relaxing several contraindications from previous guidelines (Table 112,13). Additionally, it recommends that women be transferred, as early in pregnancy as possible, to facilities that can provide TOLAC. The guideline states that women should not be forced to have a repeat cesarean delivery or be denied care if they desire TOLAC. Unfortunately, many women still may not have a practical option for TOLAC because the ACOG guideline, despite NIH recommendations, did not remove the “immediately available” stipulation.
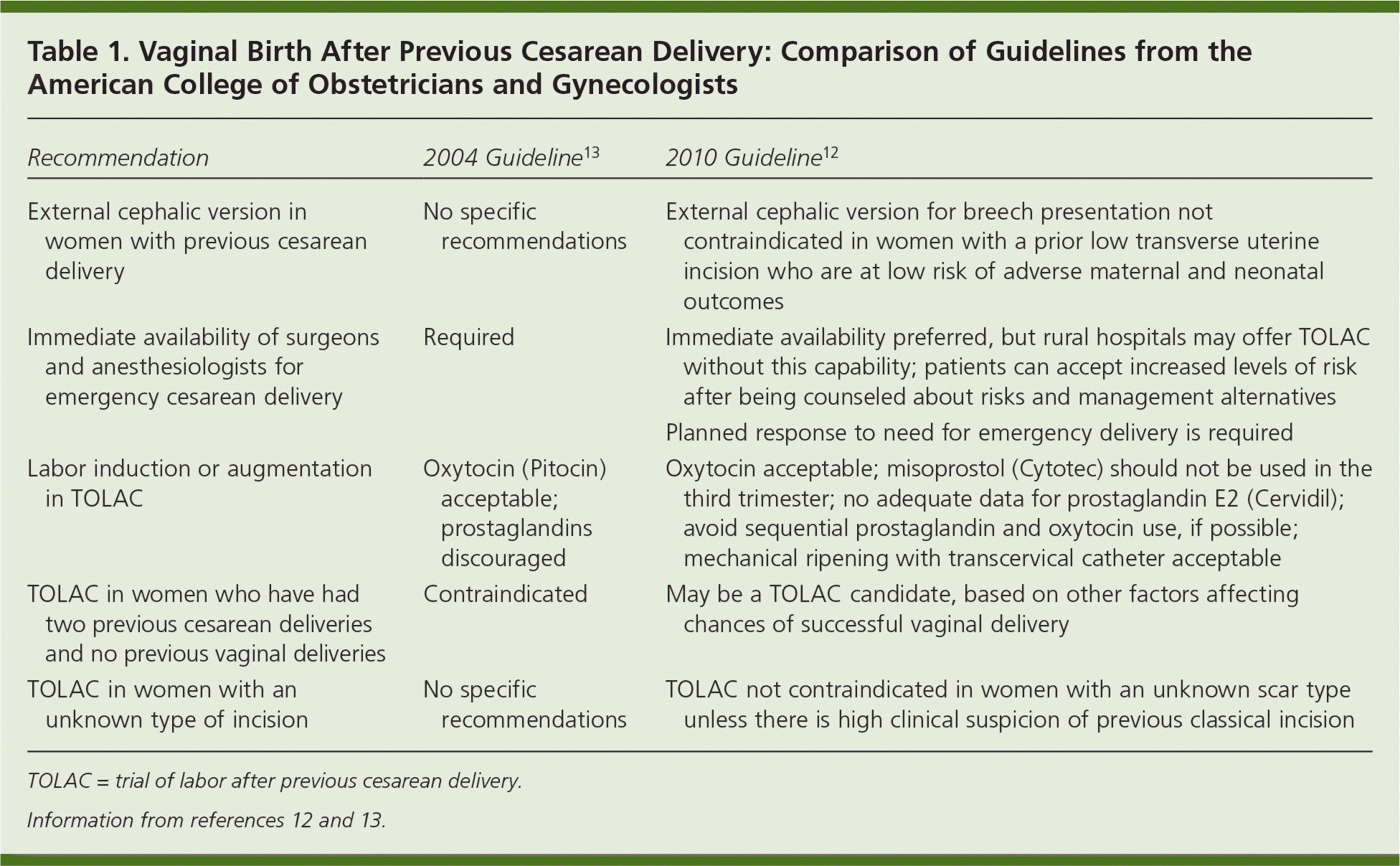
| Recommendation | 2004 Guideline13 | 2010 Guideline12 |
|---|---|---|
| External cephalic version in women with previous cesarean delivery | No specific recommendations | External cephalic version for breech presentation not contraindicated in women with a prior low transverse uterine incision who are at low risk of adverse maternal and neonatal outcomes |
| Immediate availability of surgeons and anesthesiologists for emergency cesarean delivery | Required | Immediate availability preferred, but rural hospitals may offer TOLAC without this capability; patients can accept increased levels of risk after being counseled about risks and management alternatives |
| Planned response to need for emergency delivery is required | ||
| Labor induction or augmentation in TOLAC | Oxytocin (Pitocin) acceptable; prostaglandins discouraged | Oxytocin acceptable; misoprostol (Cytotec) should not be used in the third trimester; no adequate data for prostaglandin E2 (Cervidil); avoid sequential prostaglandin and oxytocin use, if possible; mechanical ripening with transcervical catheter acceptable |
| TOLAC in women who have had two previous cesarean deliveries and no previous vaginal deliveries | Contraindicated | May be a TOLAC candidate, based on other factors affecting chances of successful vaginal delivery |
| TOLAC in women with an unknown type of incision | No specific recommendations | TOLAC not contraindicated in women with an unknown scar type unless there is high clinical suspicion of previous classical incision |
We encourage maternity care providers and hospitals that do not currently offer TOLAC to use the NIH statement and revised ACOG guidelines as an opportunity to reevaluate their policies on TOLAC. The Northern New England Perinatal Quality Improvement Network's VBAC project is an example of a collaborative effort between community hospitals and maternity care providers to develop risk-stratification guidelines and to facilitate planning for emergent cesarean delivery.14 Counseling patients about delivery options involves consideration of maternal and perinatal risks and benefits (Table 215 ), future childbearing plans, and the likelihood of successful VBAC. Most women who have had a previous cesarean delivery are candidates for TOLAC and should be offered that option. Women who have also had a previous vaginal delivery are at lower risk of uterine rupture, have a high likelihood of successful VBAC, and should be actively encouraged to consider TOLAC.16,17 Physicians should counsel women who plan to have subsequent pregnancies about the increased incidence of abnormal placentation with additional cesarean deliveries, and encourage them to consider TOLAC.10,18
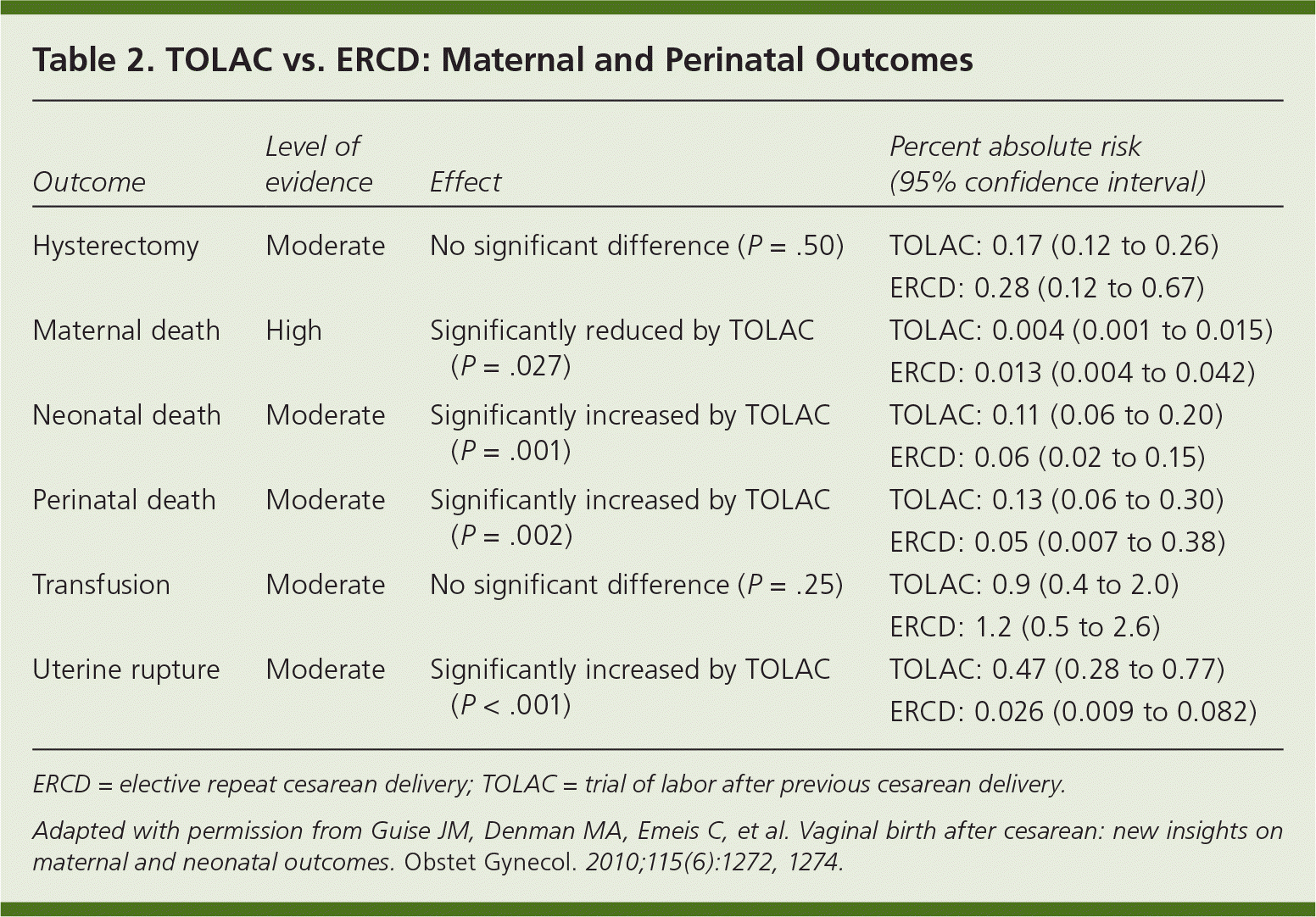
| Outcome | Level of evidence | Effect | Percent absolute risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy | Moderate | No significant difference (P =.50) | TOLAC: 0.17 (0.12 to 0.26) |
| ERCD: 0.28 (0.12 to 0.67) | |||
| Maternal death | High | Significantly reduced by TOLAC (P =.027) | TOLAC: 0.004 (0.001 to 0.015) |
| ERCD: 0.013 (0.004 to 0.042) | |||
| Neonatal death | Moderate | Significantly increased by TOLAC (P =.001) | TOLAC: 0.11 (0.06 to 0.20) |
| ERCD: 0.06 (0.02 to 0.15) | |||
| Perinatal death | Moderate | Significantly increased by TOLAC (P =.002) | TOLAC: 0.13 (0.06 to 0.30) |
| ERCD: 0.05 (0.007 to 0.38) | |||
| Transfusion | Moderate | No significant difference (P =.25) | TOLAC: 0.9 (0.4 to 2.0) |
| ERCD: 1.2 (0.5 to 2.6) | |||
| Uterine rupture | Moderate | Significantly increased by TOLAC (P <.001) | TOLAC: 0.47 (0.28 to 0.77) |
| ERCD: 0.026 (0.009 to 0.082) |
Preventing sequelae from cesarean delivery includes efforts to prevent primary cesarean delivery with evidence-based maternity care practices, such as increased use of intermittent monitoring, avoidance of elective labor induction, and the use of doulas for labor support. The American Academy of Family Physicians Board of Directors recently approved plans to revise its VBAC guideline, incorporating the evidence report from the Agency for Healthcare Research and Quality 2 and the NIH consensus statement.1 We believe the new guideline will further assist rural hospitals and family physicians as they work toward decreasing the cesarean delivery rate in the United States.
