
Am Fam Physician. 2011;83(3):293-300
A more recent article on recurrent venous thromboembolism is available.
Patient information: See related handout on venous thromboembolism, written by the authors of this article.
Author disclosure: Nothing to disclose.
A previous venous thromboembolism is the most important risk factor for predicting recurrence of the condition. Several studies have shown that routine testing for inherited thrombophilias is not helpful in predicting the risk of recurrence or altering treatment decisions, and therefore is not cost-effective. Updated practice guidelines from the American College of Chest Physicians shift the focus away from laboratory testing and place stronger emphasis on identifying clinical factors when making treatment decisions. The major determinants for treatment duration are whether the deep venous thrombosis was located in a distal or proximal vein, whether the thrombotic episode was an initial or recurrent event, and whether transient risk factors were present. Persistent elevations on the d-dimer test or the presence of residual thrombosis may provide further information to predict recurrence risk and determine treatment duration. Screening for antiphospholipid syndrome and/or malignancy should be considered in patients presenting with arterial thrombosis, thrombosis at an unusual site, or recurrent pregnancy loss. Patients with venous thromboembolism and a known malignancy should be treated with low-molecular-weight heparin rather than oral anticoagulation as long as the cancer is active. All patients with recurrent, unprovoked venous thromboembolism should be considered for long-term treatment.
The annual incidence of venous thromboembolism (VTE), which includes deep venous thrombosis and pulmonary embolism, is one or two per 1,000 persons.1–3 Recurrent thrombosis is relatively common, particularly in patients with idiopathic VTE; a previous VTE is the main risk factor for a second VTE.1–3 Following treatment of an initial thrombotic event, it is important to determine whether the VTE was provoked (acquired risk factor) or unprovoked (idiopathic) to guide duration of anticoagulant therapy.4 If a patient has a recurrent or idiopathic VTE, a careful evaluation for intrinsic risk factors should be performed.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Patients with a transient provoking risk factor, but no persistent risk factors, for VTE do not require further testing. | C | 3, 11–13 |
| Routine testing for hereditary thrombophilias in patients with a first VTE is not helpful in predicting risk of recurrence or altering initial therapy. | C | 3, 4, 11, 12, 19 |
| Extensive screening for occult malignancy in patients with VTE has not been proven to be cost-effective, to reduce mortality, or to improve survival. | B | 33, 36, 38 |
| Clinical factors, such as whether the deep venous thrombosis was confined to a distal or proximal vein, whether the thrombotic episode was an initial or recurrent event, or whether transient risk factors were present, should determine duration of anticoagulant therapy in patients with VTE. | B | 1, 4 |
| Patients with a VTE and cancer should be treated with low-molecular-weight heparin for at least the first three to six months of long-term anticoagulation therapy. Subsequent treatment with low-molecular-weight heparin or vitamin K antagonist should be continued for as long as the cancer is active. | B | 4, 39 |
Assessing Risk of Recurrent VTE
A thorough history in a patient with thrombosis should include age at the first thrombotic event, location of the thrombosis, and presence of any precipitating or provoking conditions. Risk factors for venous thromboembolism are listed in Table 1,1,3–8 and Table 2 includes the relative risk of recurrent VTE based on risk factors.9,10 A VTE is considered provoked if transient risk factors are present. These transient risk factors are divided into major and minor categories.1,3,4 The more significant the provoking risk factor (e.g., surgery, trauma), the lower the expected risk of recurrence after anticoagulant therapy is discontinued.1,4,11 Patients with a transient provoking risk factor but no persistent risk factors do not require further testing,11–13 because these patients do not have a higher risk of recurrence than the general population.1,3 Conversely, patients with idiopathic VTE are at high risk of recurrence. One study found the cumulative risk of recurrence at one, five, and 10 years to be 15, 41, and 53 percent, respectively, in patients with an idiopathic VTE, compared with 7, 16, and 23 percent in patients with a provoked event.14 Another study found the risk of recurrence to be 4.8 percent at two years in patients with transient risk factors versus 12.1 percent in those with an unprovoked event.15
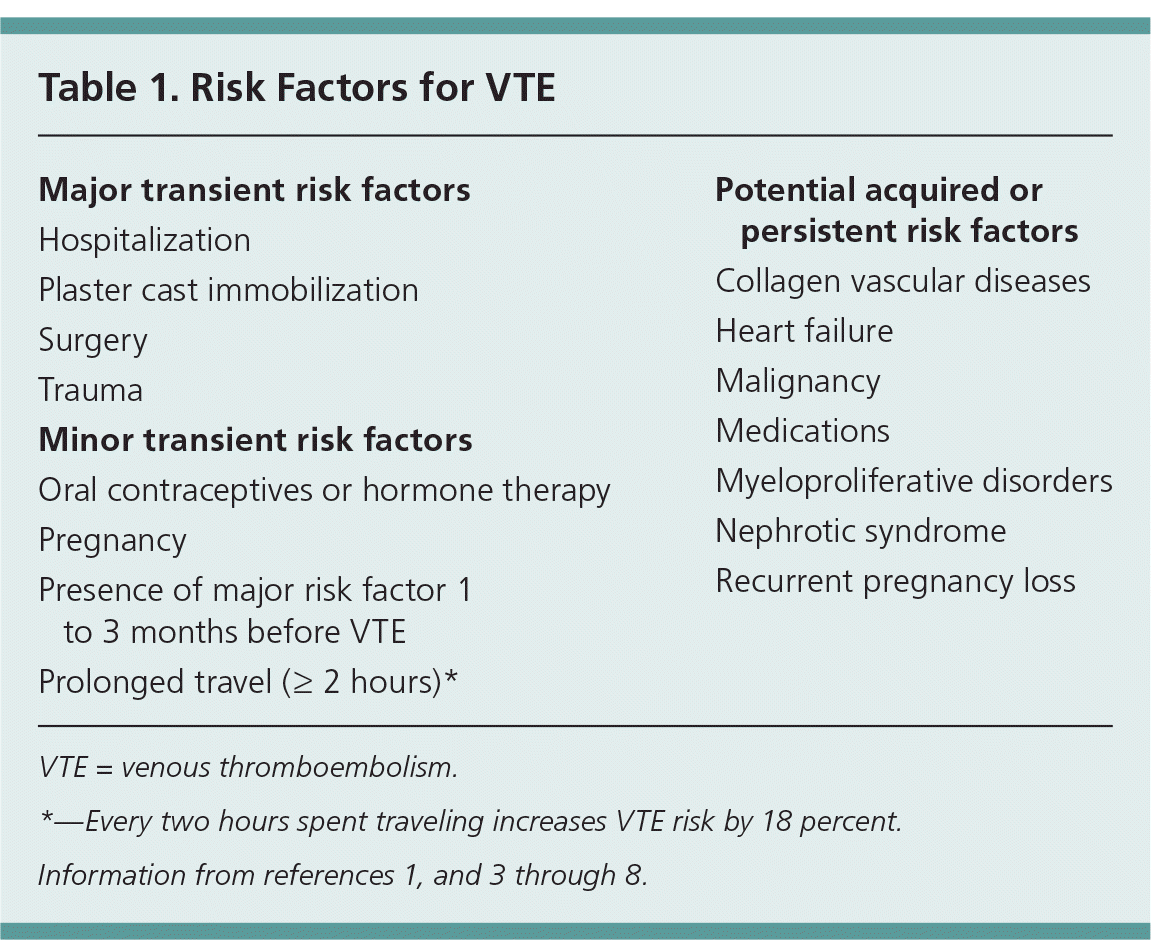
| Major transient risk factors |
| Hospitalization |
| Plaster cast immobilization |
| Surgery |
| Trauma |
| Minor transient risk factors |
| Oral contraceptives or hormone therapy |
| Pregnancy |
| Presence of major risk factor 1 to 3 months before VTE |
| Prolonged travel (≥ 2 hours)* |
| Potential acquired or persistent risk factors |
| Collagen vascular diseases |
| Heart failure |
| Malignancy |
| Medications |
| Myeloproliferative disorders |
| Nephrotic syndrome |
| Recurrent pregnancy loss |
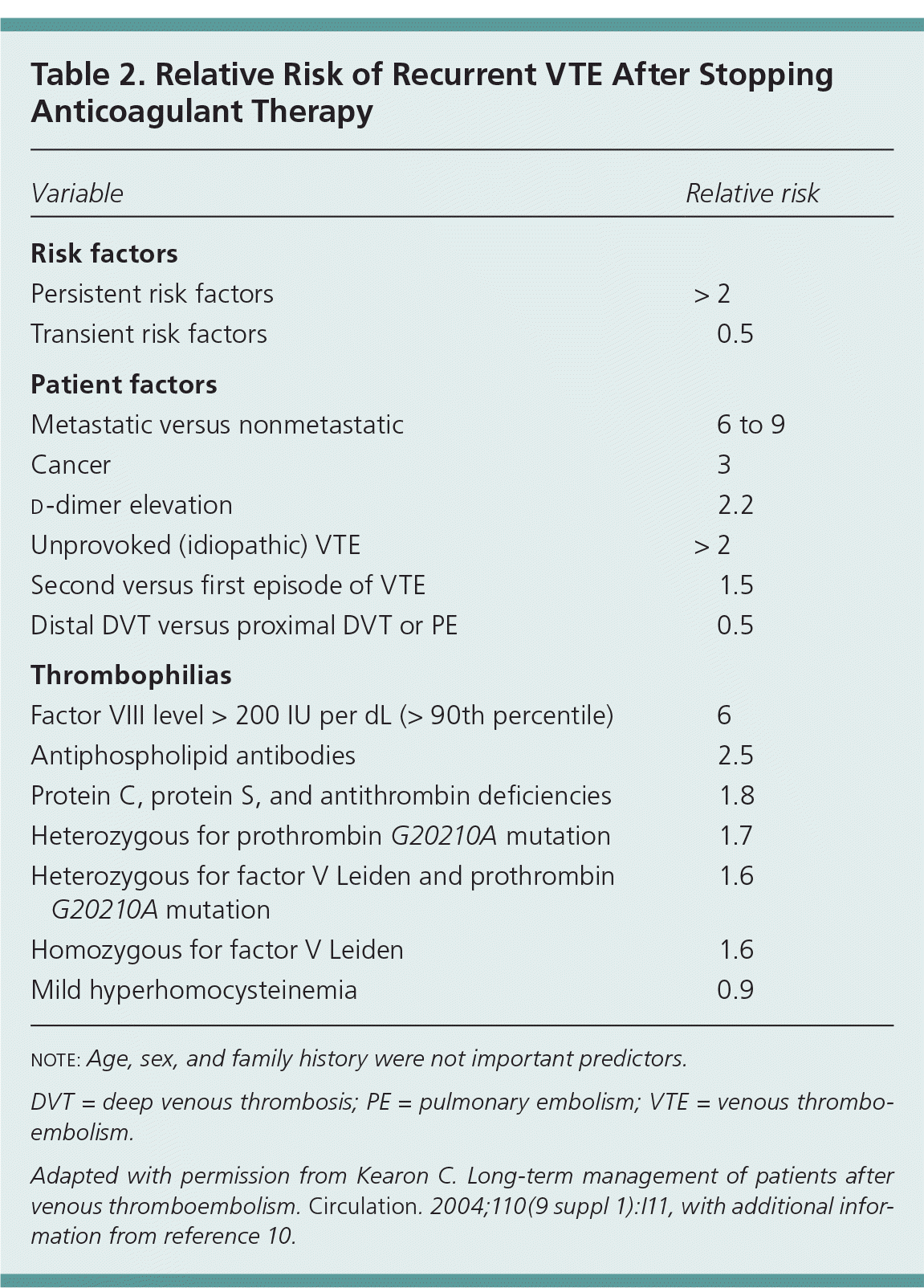
| Variable | Relative risk |
|---|---|
| Risk factors | |
| Persistent risk factors | > 2 |
| Transient risk factors | 0.5 |
| Patient factors | |
| Metastatic versus nonmetastatic | 6 to 9 |
| Cancer | 3 |
| d-dimer elevation | 2.2 |
| Unprovoked (idiopathic) VTE | > 2 |
| Second versus first episode of VTE | 1.5 |
| Distal DVT versus proximal DVT or PE | 0.5 |
| Thrombophilias | |
| Factor VIII level > 200 IU per dL (> 90th percentile) | 6 |
| Antiphospholipid antibodies | 2.5 |
| Protein C, protein S, and antithrombin deficiencies | 1.8 |
| Heterozygous for prothrombin G20210A mutation | 1.7 |
| Heterozygous for factor V Leiden and prothrombin G20210A mutation | 1.6 |
| Homozygous for factor V Leiden | 1.6 |
| Mild hyperhomocysteinemia | 0.9 |
An idiopathic VTE can be caused by an acquired or inherited thrombophilia. Table 3 includes risk factors that suggest an underlying thrombophilia.1,7,16,17 Antiphospholipid syndrome is the most common cause of acquired thrombophilia. This syndrome is usually secondary to autoimmune disease and may cause venous or arterial thrombosis, thrombocytopenia, acute ischemic encephalopathy, or recurrent pregnancy loss.7,18 Antiphospholipid syndrome may also be induced by the use of certain medications, such as hydralazine, phenothiazines, or procainamide. Other thrombophilias include factor V Leiden deficiency, prothrombin G20210A mutation, antithrombin deficiency, and protein C and protein S deficiency. 1,3,11,12 Elevated levels of homocysteine, factor VIII, factor IX, and factor XI may also increase the risk of VTE.1,3,11,12,19
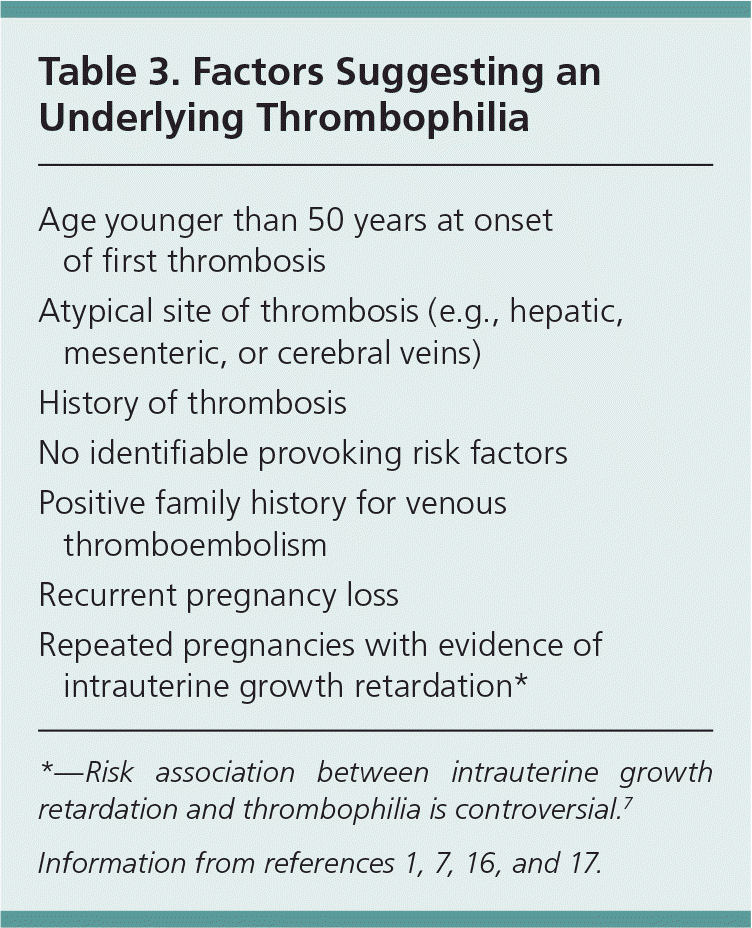
| Age younger than 50 years at onset of first thrombosis |
| Atypical site of thrombosis (e.g., hepatic, mesenteric, or cerebral veins) |
| History of thrombosis |
| No identifiable provoking risk factors |
| Positive family history for venous thromboembolism |
| Recurrent pregnancy loss |
| Repeated pregnancies with evidence of intrauterine growth retardation* |
There is no consensus regarding who should be tested for inherited thrombophilias, 20,21 and several studies have called into question the cost-effectiveness of routinely testing patients with an initial idiopathic VTE.3,11,12 Routine testing has not been shown to be helpful in predicting risk of recurrence, deciding the duration of initial treatment, or determining the need for long-term prophylactic anticoagulation.3,11,12 A systematic review examining the risk of recurrence in persons with an initial idiopathic VTE found only a modest increase in risk in persons who were heterozygous for factor V Leiden or who had a prothrombin gene mutation. The difference between those with and those without either of these conditions was not significant, and patients did not benefit significantly from an extended period of anticoagulant therapy.12 An evidence report prepared for the Agency for Healthcare Research and Quality (AHRQ) on genetic testing in patients with a history of VTE found only low-grade evidence (derived from models) that testing for factor V Leiden, prothrombin G20210A mutation, or both is cost-effective when caring for patients with VTE or their family members. 19 The AHRQ report also found low-grade evidence that these test results altered patient management decisions, and no direct evidence that testing leads to improved clinical outcomes, such as reduced incidence of recurrent VTE.
Updated guidelines from the American College of Chest Physicians (ACCP) shift the focus away from testing for the presence of a thrombophilia to assessing the risk of recurrent VTE based on location of the thrombus, whether it was idiopathic, and whether it was recurrent when considering treatment duration.4 Thrombophilia testing should be considered only if it is clear that the results would influence management decisions. For patients who have had a VTE, the knowledge of thrombophilia does not seem to have any specific impact on future management decisions, with the possible exception of antiphospholipid syndrome in pregnancy.7,10 Table 4 summarizes guidelines for prevention of recurrent VTE in pregnancy.7,22
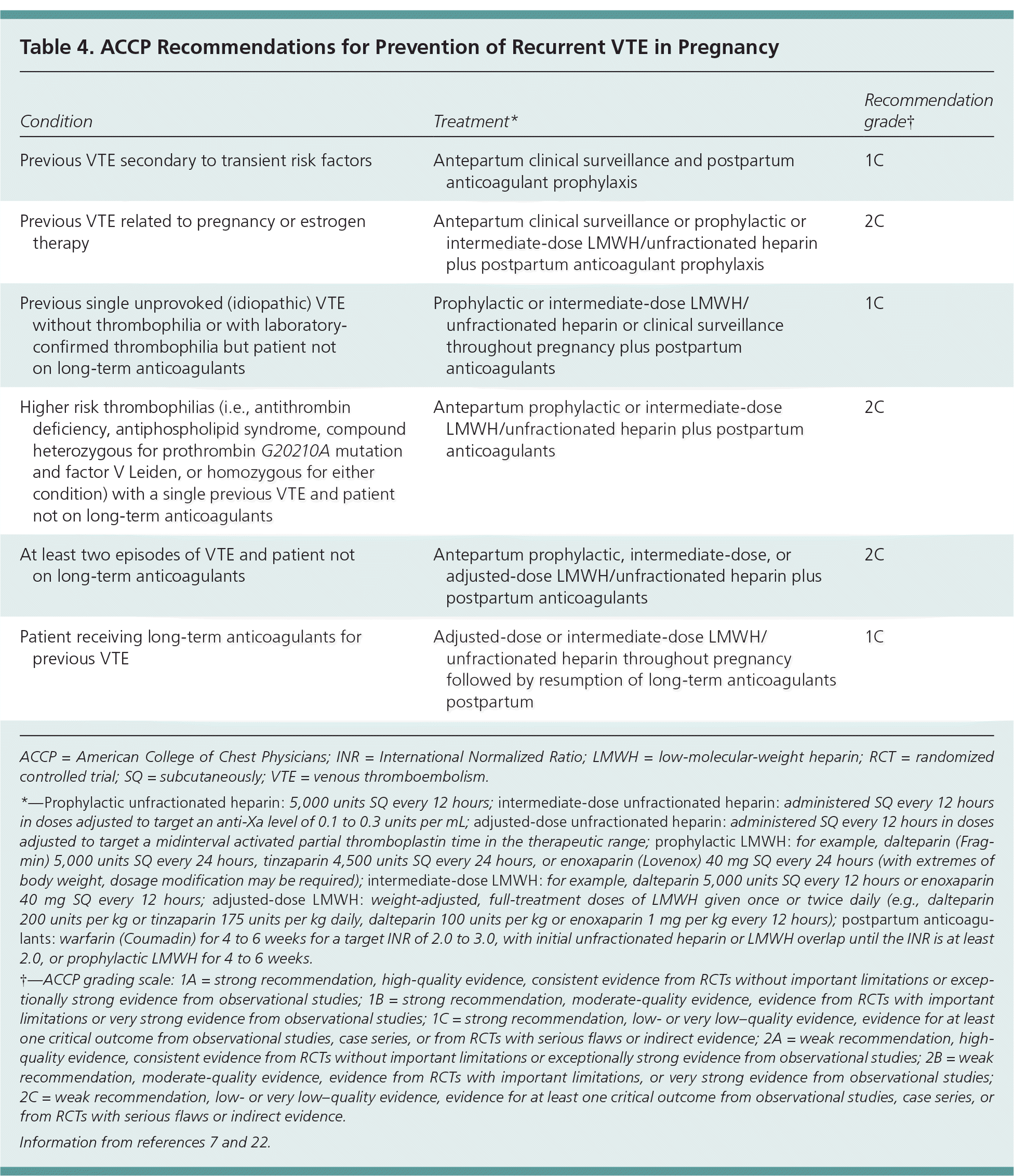
| Condition | Treatment* | Recommendation grade† |
|---|---|---|
| Previous VTE secondary to transient risk factors | Antepartum clinical surveillance and postpartum anticoagulant prophylaxis | 1C |
| Previous VTE related to pregnancy or estrogen therapy | Antepartum clinical surveillance or prophylactic or intermediate-dose LMWH/unfractionated heparin plus postpartum anticoagulant prophylaxis | 2C |
| Previous single unprovoked (idiopathic) VTE without thrombophilia or with laboratory-confirmed thrombophilia but patient not on long-term anticoagulants | Prophylactic or intermediate-dose LMWH/unfractionated heparin or clinical surveillance throughout pregnancy plus postpartum anticoagulants | 1C |
| Higher risk thrombophilias (i.e., antithrombin deficiency, antiphospholipid syndrome, compound heterozygous for prothrombin G20210A mutation and factor V Leiden, or homozygous for either condition) with a single previous VTE and patient not on long-term anticoagulants | Antepartum prophylactic or intermediate-dose LMWH/unfractionated heparin plus postpartum anticoagulants | 2C |
| At least two episodes of VTE and patient not on long-term anticoagulants | Antepartum prophylactic, intermediate-dose, or adjusted-dose LMWH/unfractionated heparin plus postpartum anticoagulants | 2C |
| Patient receiving long-term anticoagulants for previous VTE | Adjusted-dose or intermediate-dose LMWH/unfractionated heparin throughout pregnancy followed by resumption of long-term anticoagulants postpartum | 1C |
Laboratory and Other Testing
Before the initiation of anticoagulant therapy, certain baseline laboratory studies should be ordered to confirm that anticoagulation would be safe for the patient (Table 5).5,23–26 Impaired liver or renal function may require adjustments to anticoagulant dosing. 23,24 Laboratory testing may also identify potential persistent risk factors for recurrent thrombosis. For example, elevations in the hematocrit or platelet count, especially if splenomegaly is present, can suggest a myeloproliferative disorder 1,5; polycythemia or thrombocytosis may suggest an underlying occult malignancy; prolongation of the partial thromboplastin time that is not corrected using a 1:1 dilution with normal plasma may suggest lupus anticoagulant syndrome 25; and high levels of urine protein may suggest nephrotic syndrome.6,26
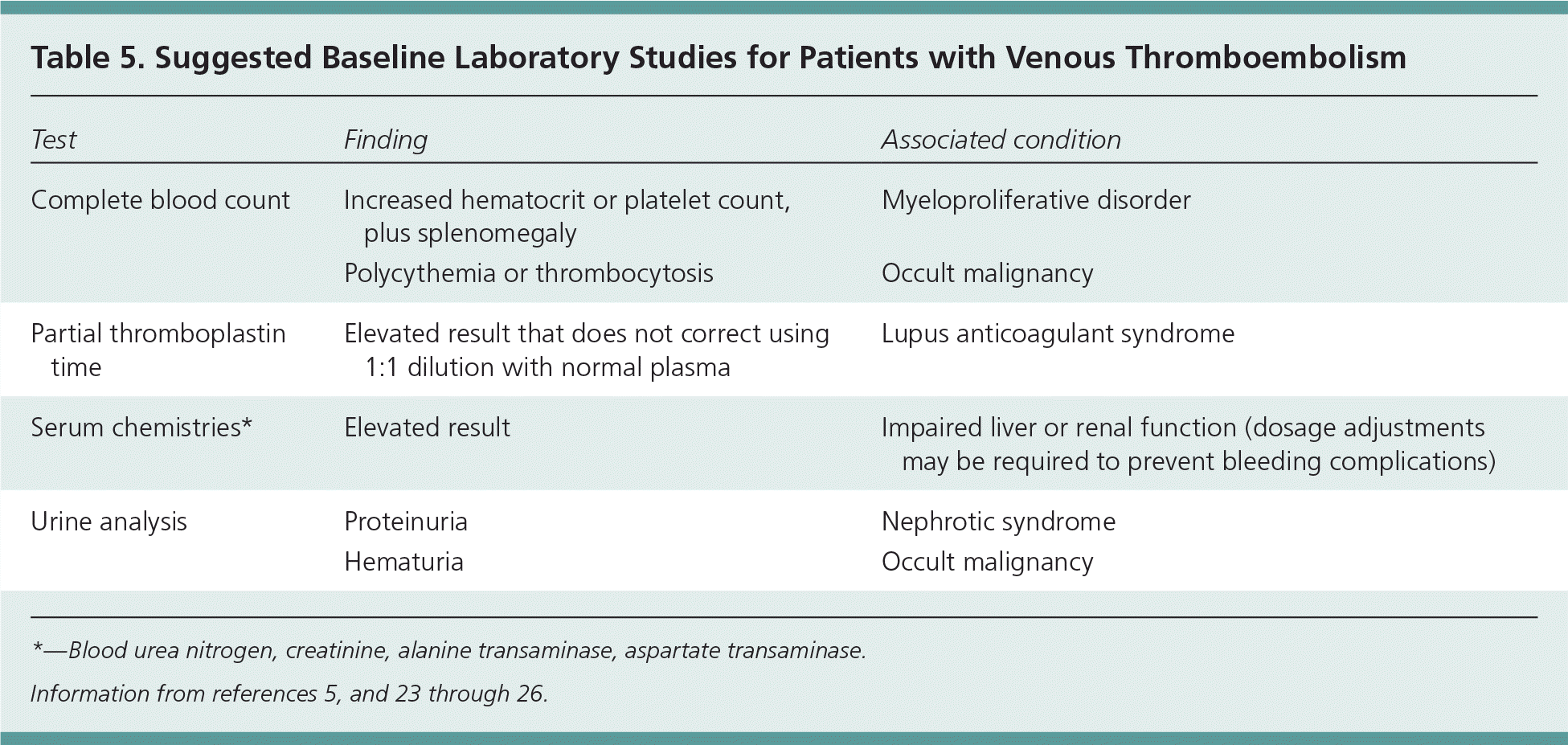
| Test | Finding | Associated condition |
|---|---|---|
| Complete blood count | Increased hematocrit or platelet count, plus splenomegaly | Myeloproliferative disorder |
| Polycythemia or thrombocytosis | Occult malignancy | |
| Partial thromboplastin time | Elevated result that does not correct using 1:1 dilution with normal plasma | Lupus anticoagulant syndrome |
| Serum chemistries* | Elevated result | Impaired liver or renal function (dosage adjustments may be required to prevent bleeding complications) |
| Urine analysis | Proteinuria | Nephrotic syndrome |
| Hematuria | Occult malignancy |
Recent studies have also identified other laboratory and imaging tests that can help predict recurrence or decide treatment duration. A persistently elevated d-dimer value one month after stopping anticoagulation has been associated with an increased risk of recurrent VTE. A recent systematic review found that patients with a negative d-dimer result after at least three months of anticoagulation had an annual recurrence rate of 3.5 percent, compared with 8.9 percent in those with a persistently elevated d-dimer result.29 In another study, the presence of residual thrombosis on ultrasonography after anticoagulation was associated with a significant risk of recurrence.30 However, the PROLONG study found that the presence of residual venous occlusion was not a risk factor for VTE recurrence,31 but confirmed that an elevated d-dimer result one month after anticoagulation withdrawal was a risk factor for VTE recurrence.31 Although d-dimer measurement after the cessation of anticoagulation is a promising tool, it has not yet been incorporated into practice guidelines.
Timing of Testing
The thrombotic event itself or treatment with heparin or warfarin (Coumadin) can influence the results of assays for thrombophilias and d-dimer testing to assess the risk of recurrence. Systemic conditions, such as acute inflammatory response processes, liver failure, nephrotic syndrome, and disseminated intravascular coagulation, can also affect these tests. Therefore, measurement of these functional assays should be postponed until the thrombotic event is resolved and anticoagulant therapy has been discontinued for three to four weeks.30 Because antiphospholipid antibodies are acquired and may be transient, these laboratory tests should be repeated at least once 12 weeks after an initial positive result to confirm the diagnosis.32 The notable exception is genetic testing for factor V Leiden and prothrombin G20210A mutation, which can be ordered at any time.12,13
Evaluation for Malignancy
VTE may be the first manifestation of an underlying occult malignancy or may indicate recurrence of a previously treated cancer. A systematic review found that approximately 10 percent of patients who presented with an unprovoked or idiopathic VTE received a cancer diagnosis within one year of the thrombotic event.33 An unprovoked VTE is most commonly associated with pancreatic, lung, and gastrointestinal cancers. Other associated malignancies include prostate, ovarian, and brain cancers, lymphoma, and acute leukemia.34,35 Therefore, patients should be asked about history of cancer or constitutional symptoms that may suggest an underlying malignancy (e.g., loss of appetite, weight loss, fatigue, pain, hematochezia, hemoptysis, hematuria). In addition to a thorough history, a complete physical examination should be performed, including colorectal cancer screening and a pelvic examination in women.36 However, detection of an occult malignancy is clinically important only if it leads to improved survival, which often is not the case if the malignancy has metastasized and is causing constitutional symptoms.34
Data from the Computerized Registry of Patients with Venous Thromboembolism (RIETE registry) found that occult malignancy was more common in patients 60 to 75 years of age and in those with idiopathic VTE, bilateral deep venous thrombosis, or anemia.37 Other recent reviews have compared the benefits of limited versus extensive cancer screening protocols. In most of the studies, limited screening involved a history, physical examination, laboratory blood testing (i.e., complete blood count, electrolyte levels, creatinine level, calcium level, liver function tests), urinalysis, and chest radiography. 38 Extensive screening included limited screening plus ultrasonography or computed tomography of the abdomen and pelvis, and measurement of tumor markers (e.g., prostate-specific antigen, carcinoembryonic antigen, cancer antigen 125).38 Of the 10 percent of patients with cancer, approximately one-half could be identified with limited screening.36,38 The extensive screening protocol increased this detection rate to 67 percent.38 However, these studies did not determine whether increased detection through extensive screening is cost-effective, reduces morbidity, or improves survival.33,36,38 Because the clinical usefulness of extensive screening has not been established, only limited screening for malignancy can be recommended in patients with idiopathic VTE.
Duration of Therapy
The ACCP guidelines on antithrombotic and thrombolytic therapy do not recommend testing for the presence of a hereditary thrombophilia to guide decisions on the duration of anticoagulant therapy.4 This is based on data from several prospective studies that suggest that results of this testing are not major determinants in predicting the risk of recurrence. Instead, the guidelines recommend using clinical factors, such as whether the deep venous thrombosis was confined to a distal or proximal vein, whether the thrombotic episode was an initial or recurrent episode, and whether transient risk factors were present.1,4 Table 6 summarizes the ACCP guidelines on duration of anticoagulant therapy.4,22 Patients with known malignancy should be treated with low-molecular-weight heparin, as long as the cancer is active.39,40 All other patients, regardless of clinical factors, should receive at least three months of anticoagulant therapy.40 However, the optimal duration of therapy depends on balancing the risk of recurrence, the risk of major hemorrhage (approximately 1 percent per year in low-risk patients4), and the cost and inconvenience of anticoagulation. The d-dimer test shows promise in guiding treatment duration, but further refinements are needed before such testing is routine.
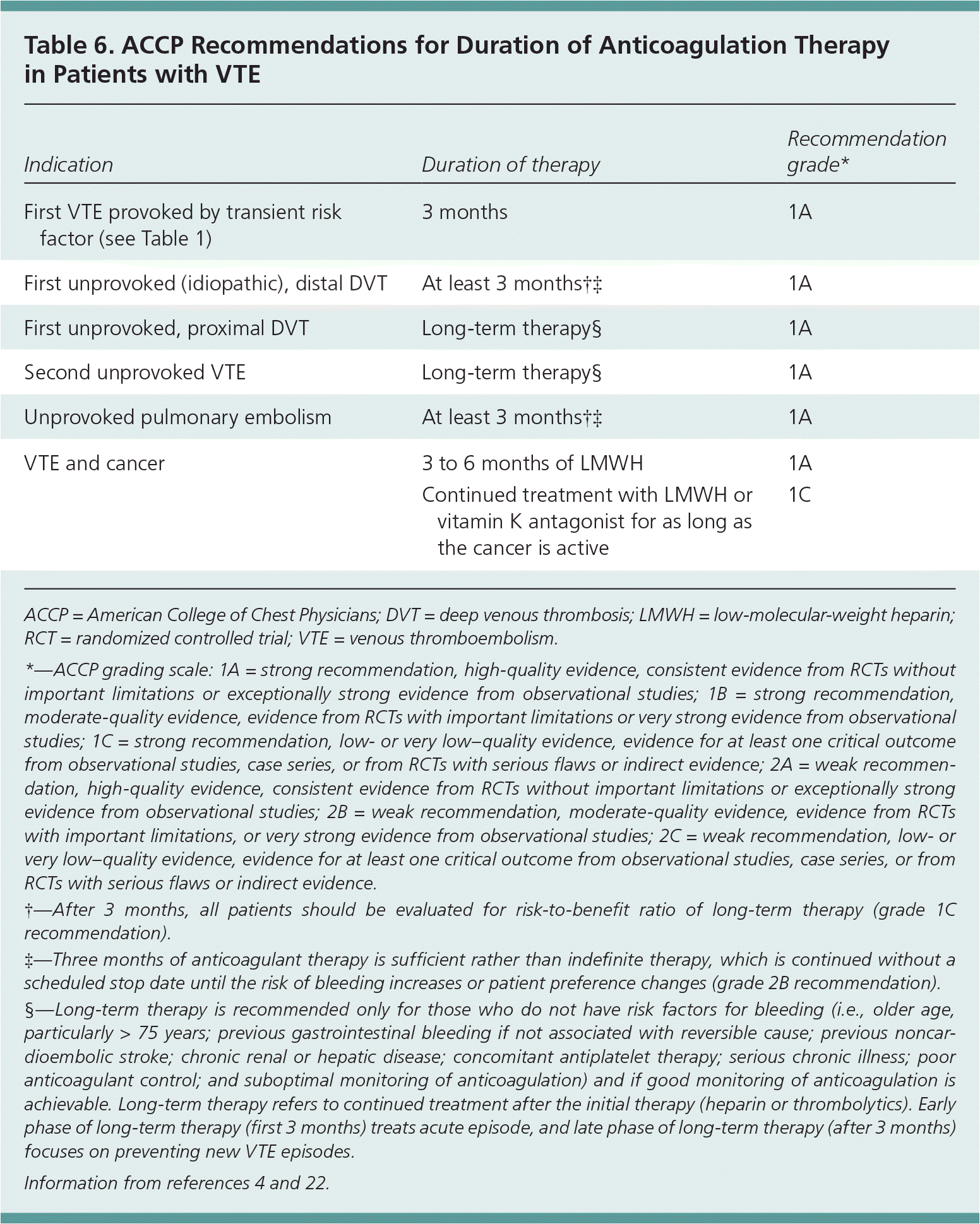
| Indication | Duration of therapy | Recommendation grade* |
|---|---|---|
| First VTE provoked by transient risk factor (see Table 1) | 3 months | 1A |
| First unprovoked (idiopathic), distal DVT | At least 3 months†‡ | 1A |
| First unprovoked, proximal DVT | Long-term therapy§ | 1A |
| Second unprovoked VTE | Long-term therapy§ | 1A |
| Unprovoked pulmonary embolism | At least 3 months†‡ | 1A |
| VTE and cancer | 3 to 6 months of LMWH | 1A |
| Continued treatment with LMWH or vitamin K antagonist for as long as the cancer is active | 1C |
