Am Fam Physician. 2011;83(4):464-466
Author disclosure: Nothing to disclose.
Clinical Question
What treatments are effective for excessive sweating (i.e., hyperhidrosis)?
Evidence-Based Answer
OnabotulinumtoxinA injections (Botox; formerly botulinum toxin type A) are effective for the treatment of primary axillary and palmar hyperhidrosis. (Strength of Recommendation [SOR]: A, based on a systematic review of randomized controlled trials). Topical aluminum chloride (Drysol) is effective for the treatment of palmar hyperhidrosis. (SOR: B, based on one small controlled study). Iontophoresis is effective for treating palmar, plantar, and axillary hyperhidrosis. (SOR: C, based on one unblinded, randomized controlled trial). There are no identified randomized, placebo-controlled trials of thoracoscopic sympathectomy or systemic medical therapy for the treatment of hyperhidrosis.
Evidence Summary
Hyperhidrosis is a common disorder, affecting an estimated 2.8 percent of the U.S. population.1 Focal hyperhidrosis most commonly affects the axillae, palms, and soles. OnabotulinumtoxinA injections, topical aluminum chloride, and iontophoresis have been investigated for treating focal and multifocal hyperhidrosis. There have been only case series of thoracoscopic sympathectomy or systemic medical therapy for the treatment of hyperhidrosis.
A systematic review assessing the effectiveness of onabotulinumtoxinA injection for a variety of conditions, including hyperhidrosis, concluded that it is effective for the treatment of axillary hyperhidrosis and likely effective for palmar hyperhidrosis.2 Five randomized controlled trials consistently found that onabotulinumtoxinA injections reduced sweat production or symptoms (Table 1).3–7 OnabotulinumtoxinA injections have been shown to be effective for at least 16 weeks.4
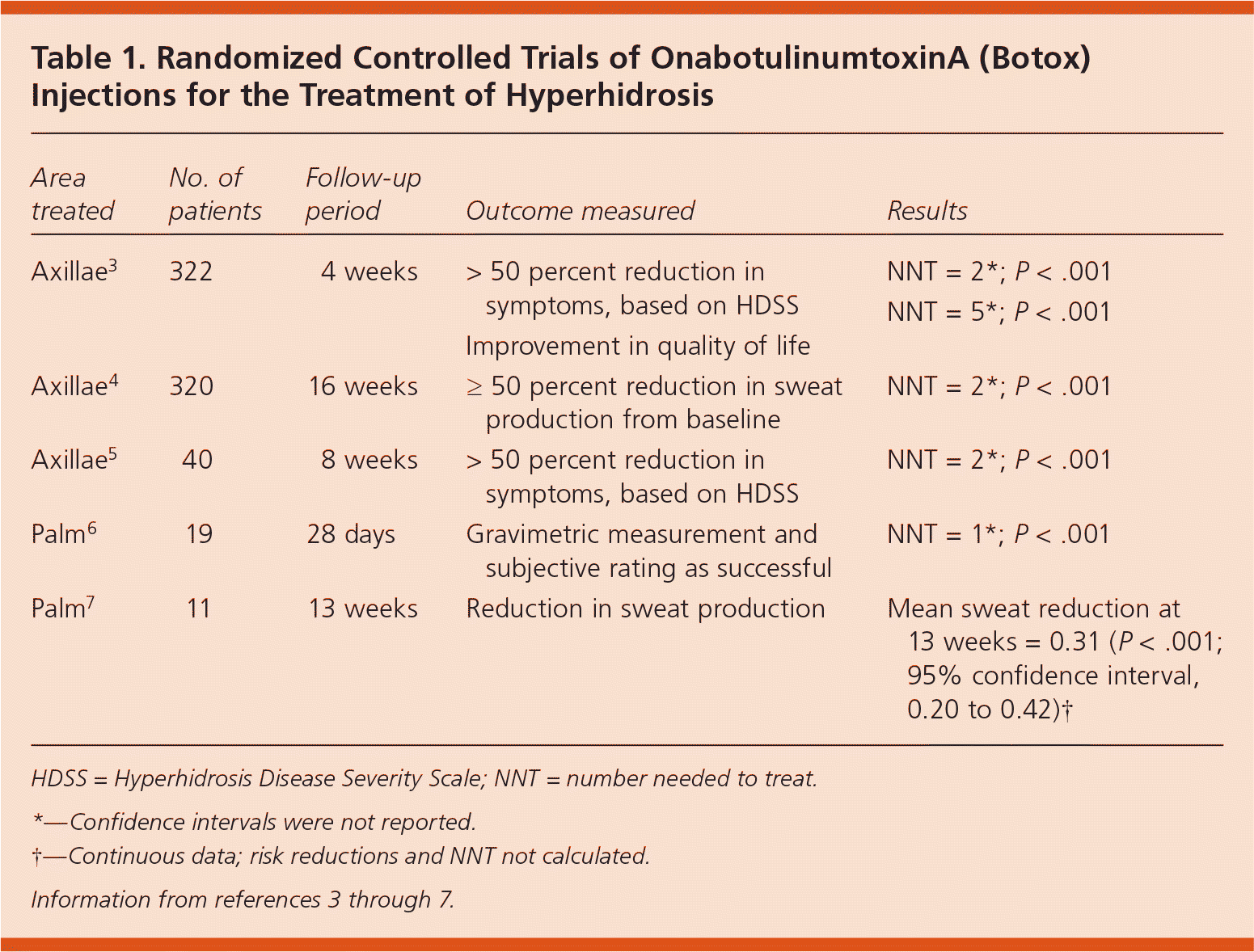
| Area treated | No. of patients | Follow-up period | Outcome measured | Results |
|---|---|---|---|---|
| Axillae3 | 322 | 4 weeks | > 50 percent reduction in symptoms, based on HDSS Improvement in quality of life | NNT = 2*; P < .001 NNT = 5*; P < .001 |
| Axillae4 | 320 | 16 weeks | ≥ 50 percent reduction in sweat production from baseline | NNT = 2*; P < .001 |
| Axillae5 | 40 | 8 weeks | > 50 percent reduction in symptoms, based on HDSS | NNT = 2*; P < .001 |
| Palm6 | 19 | 28 days | Gravimetric measurement and subjective rating as successful | NNT = 1*; P < .001 |
| Palm7 | 11 | 13 weeks | Reduction in sweat production | Mean sweat reduction at 13 weeks = 0.31 (P < .001; 95% confidence interval, 0.20 to 0.42)† |
Aluminum chloride may be effective for treating hyperhidrosis. In a single-blind trial of 12 Chinese men diagnosed with palmar hyperhidrosis, participants used topical aluminum chloride nightly on one palm for four weeks.8 Each participant used his opposite palm as the control. Sweat vapor loss was measured with an evaporimeter. All participants had a statistically significant reduction in sweat vapor after four weeks, but returned to baseline after treatment was stopped.
Iontophoresis may be effective for treating hyperhidrosis. In a controlled study of 22 patients with focal hyperhidrosis (including the axillae, palms, and soles), one-half of participants' affected areas were treated with tap water iontophoresis.9 Patients served as their own controls, and were treated until symptoms resolved. The longest duration of treatment was 41 days. Patients were reevaluated at the final treatment and at one month after the final treatment. Sweat output was measured using Persprint paper and computer analysis. Overall, 93 percent of the affected areas had responded to treatment by day 20.
A single-blind, right-left comparison study of 20 patients with palmoplantar hyperhidrosis compared tap water iontophoresis with glycopyrrolate iontophoresis. In patients treated with glycopyrrolate iontophoresis, the median number of days of self-reported hand dryness was 11, compared with three days for those treated with tap water iontophoresis (P < .0001).10
Recommendations from Others
The Canadian Hyperhidrosis Advisory Committee recommends topical aluminum chloride in a concentration of 20 to 50 percent for treating mild focal or multifocal hyperhidrosis.11 For patients with moderate to severe hyperhidrosis, the committee recommends starting treatment with topical aluminum chloride, and, if ineffective, trying iontophoresis or onabotulinumtoxinA injections. Further interventions, such as surgery, should be reserved for patients who do not respond to less invasive interventions.