
Am Fam Physician. 2011;83(8):978-979
Author disclosure: Nothing to disclose.
Alvimopan (Entereg) is an oral, peripherally-acting mu-opioid receptor antagonist used to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection with primary anastomosis.1 Opioids used postoperatively may cause or prolong postoperative ileus. Alvimopan blocks the opioid effect in the gastrointestinal tract without interfering with the central-acting analgesic effects of opioids. The U.S. Food and Drug Administration (FDA) has labeled alvimopan for short-term use in hospitals.
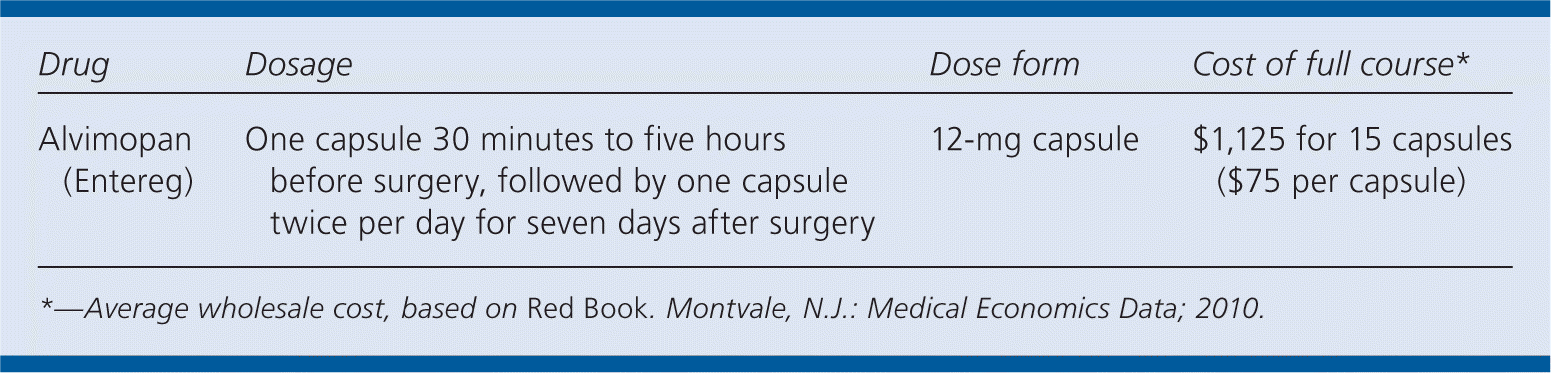
| Drug | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Alvimopan (Entereg) | One capsule 30 minutes to five hours before surgery, followed by one capsule twice per day for seven days after surgery | 12-mg capsule | $1,125 for 15 capsules ($75 per capsule) |
SAFETY
Few serious reactions have been attributed to alvimopan. Plasma levels are about 10 times higher in patients with severe hepatic impairment, although the clinical relevance of this increase is not known. Alvimopan should not be given to patients who have taken an opioid analgesic continuously for seven days or longer. Although an increased risk of myocardial infarction is associated with long-term use (i.e., 12 months), it has not been reported with treatment lasting seven days or less. No drug interactions have been identified. Alvimopan is FDA pregnancy category B; safety has not been established in breastfeeding mothers.1
TOLERABILITY
Alvimopan is generally well tolerated. In controlled studies, approximately 3 percent of patients receiving alvimopan experienced adverse effects compared with 1 percent of those receiving placebo. Adverse effects include anemia, back pain, dyspepsia, hypokalemia, and urinary retention. Alvimopan does not affect pain relief.2
EFFECTIVENESS
Alvimopan will decrease the time to hospital discharge by about one day for patients undergoing bowel resection with primary anastomosis. It will not, however, accelerate gastrointestinal recovery.3 No head-to-head studies have compared alvimopan with similar agents. Although not labeled for these uses, alvimopan has been shown to improve the time to first bowel movement in women following simple total abdominal hysterectomy, 4 and increase bowel movements in patients with opioid-induced bowel dysfunction. 2 Studies have not evaluated the effects of alvimopan on postoperative complications or mortality.
PRICE
A full seven-day course of alvimopan (15 capsules) will cost $1,125. In comparison, other medications used to increase gastrointestinal motility, such as metoclopramide (Reglan) and erythromycin, cost less than $5 for a one-week supply.
SIMPLICITY
The recommended dosage of alvimopan for treatment of postoperative ileus is 12 mg taken 30 minutes to five hours before surgery, followed by 12 mg twice per day for a maximum of 15 doses or until hospital discharge. Alvimopan can be taken with or without food.1 To ensure short-term use, sale is restricted only to hospitals that enroll in the Entereg Access Support and Education program.
Bottom Line
Use of alvimopan should be restricted to patients at a higher risk of postoperative ileus following abdominal surgeries, such as hospitalized patients undergoing bowel resection with primary anastomosis. It is an expensive option with limited indications; head-to-head studies with similar agents are needed. Alvimopan is not available for the treatment of nonhospitalized patients.
