
Am Fam Physician. 2011;83(10):1140-1143
This is one in a series of pro/con editorials discussing controversial issues in family medicine.
Reflux is a common and challenging problem among primary care patients. This condition deserves particular attention given its association with esophageal adenocarcinoma, a disease that is rising in incidence1 and kills approximately 6,000 persons annually.2 Esophageal adenocarcinoma can occur when acid exposure to the esophageal epithelium leads to chronic esophagitis. Subsequently, metaplasia may occur, progressing to dysplasia and ultimately carcinoma. Barrett's esophagus is a precursor lesion for these cancers and can be diagnosed easily by endoscopic visualization of columnar mucosa in the distal esophageal epithelium, and can be confirmed by biopsy demonstrating intestinal metaplasia.3 A strong argument can be made for Barrett's esophagus screening to prevent esophageal adenocarcinoma because excellent screening and treatment options are available.
Despite increasing evidence supporting prevention of esophageal adenocarcinoma through endoscopic screening and surveillance for Barrett's esophagus, the effectiveness of this approach has been questioned.3 Even the most recent American College of Gastroenterology guidelines stop short of recommending endoscopic screening.4 However, screening patients at high risk of developing Barrett's esophagus and esophageal adenocarcinoma is supported by solid evidence on risk factors for Barrett's esophagus, effectiveness of chemoprevention, rates of progression from Barrett's esophagus to esophageal adenocarcinoma, and the cost-effectiveness of screening programs.
It has been estimated that endoscopic screening of all patients older than 50 years with weekly reflux symptoms would include at least 10 million patients, and that this is not cost-effective based on low rates of progression from reflux to esophageal adenocarcinoma.3 However, new data on risk stratification favor selective screening. Patients can be stratified based on known risk factors for Barrett's esophagus, including smoking, obesity (body mass index greater than 30 kg per m2), longer duration of gastroesophageal reflux disease symptoms, and other factors described in Table 1.5–10 These data are strengthened by a recent nationwide retrospective cohort study conducted in the Netherlands confirming that male sex, older age, and low-grade dysplasia at initial diagnosis are independent predictors of malignant progression in Barrett's esophagus.11 Patients with high-grade dysplasia on diagnosis of Barrett's esophagus have a 6 to 7 percent higher rate of malignant transformation per year according to a meta-analysis performed in 2008.12
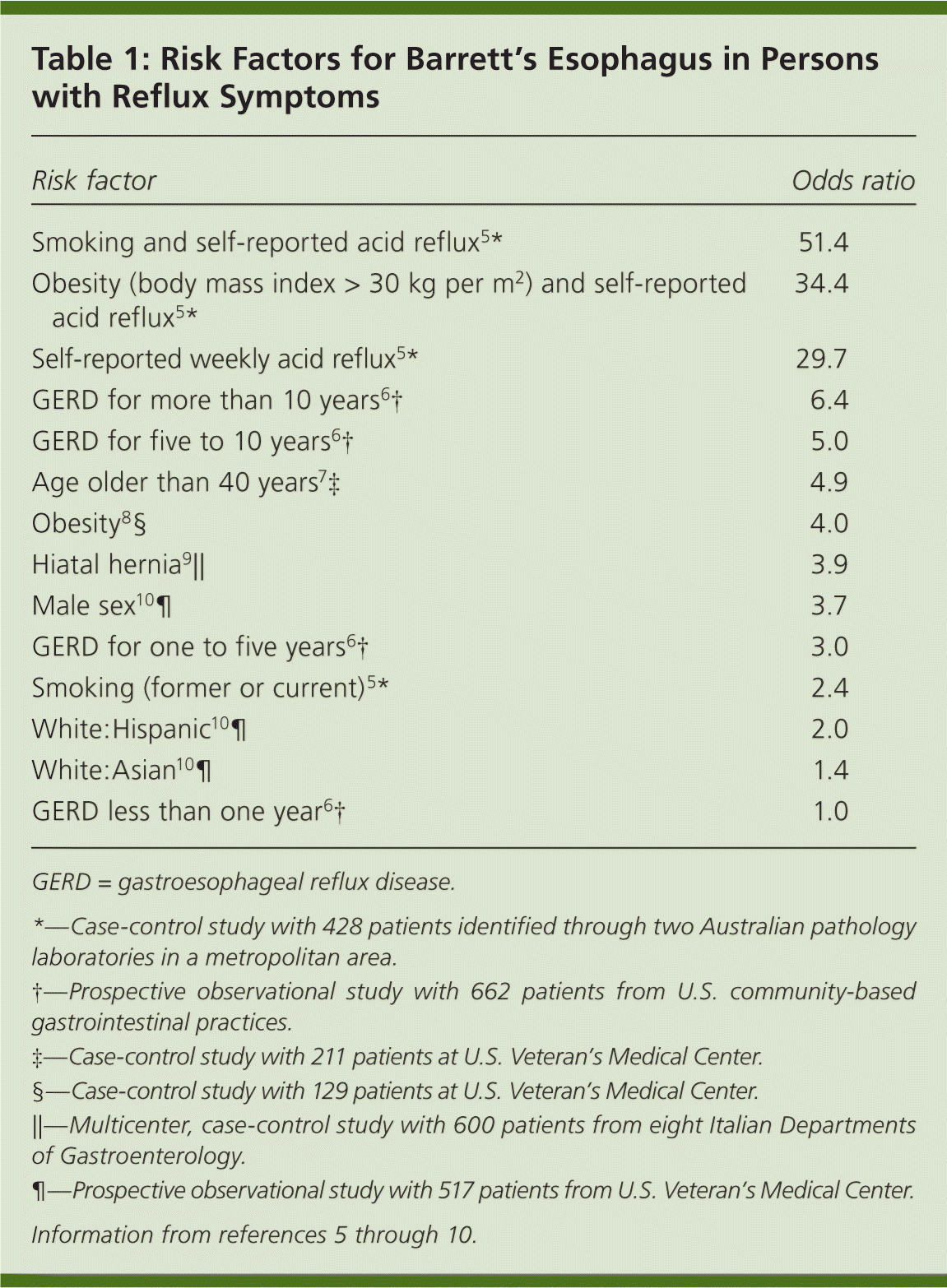
| Risk factor | Odds ratio |
|---|---|
| Smoking and self-reported acid reflux5 * | 51.4 |
| Obesity (body mass index > 30 kg per m2) and self-reported acid reflux5 * | 34.4 |
| Self-reported weekly acid reflux5 * | 29.7 |
| GERD for more than 10 years6 † | 6.4 |
| GERD for five to 10 years6 † | 5.0 |
| Age older than 40 years7 ‡ | 4.9 |
| Obesity8 § | 4.0 |
| Hiatal hernia9 ∥ | 3.9 |
| Male sex10 ¶ | 3.7 |
| GERD for one to five years6 † | 3.0 |
| Smoking (former or current)5 * | 2.4 |
| White:Hispanic10 ¶ | 2.0 |
| White:Asian10 ¶ | 1.4 |
| GERD less than one year6 † | 1.0 |
In patients with Barrett's esophagus and/or dysplasia, prevention efforts have advanced dramatically. Chemoprevention remains a mainstay in treatment, with growing evidence for newer endoscopic techniques and survival data supporting surveillance in patients with Barrett's esophagus. All patients with Barrett's esophagus and no dysplasia should be treated with proton pump inhibitors because there is a 37 percent absolute risk reduction for developing dysplasia (number needed to treat = 2.7) based on a 20-year prospective cohort.13 Support for endoscopic therapies includes a recent retrospective comparison study of patients with high-grade dysplasia that found no significant difference in five-year mortality between photodynamic therapy (8.5 percent) and esophagectomy (9 percent).14 Additionally, a Cochrane review that included 16 studies and 1,074 patients found good evidence that photodynamic therapy, argon plasma coagulation, and radiofrequency ablation all induced regression of Barrett's esophagus and dysplasia. Results favored radiofrequency ablation compared with photodynamic therapy because of fewer complications and effective eradication of Barrett's esophagus (82 percent) and dysplasia (94 percent).15 With surgery, operative mortality for esophagectomy can be improved by limiting this procedure to hospitals that perform at least 20 procedures annually.16
Endoscopic surveillance in patients with Barrett's esophagus is supported by multiple retrospective studies demonstrating statistically significant survival advantage in patients with cancers detected by endoscopic surveillance based on an average absolute risk reduction of 58 percent across all studies (number needed to treat = 1.7).4 According to the American College of Gastroenterology's Practice Guidelines, patients diagnosed with Barrett's esophagus (without dysplasia) should receive two upper endoscopies within one year and then every three years thereafter. More frequent intervals are recommended if dysplasia is present.4
One-time screening with targeted surveillance in white men 50 years of age with gastroesophageal reflux disease is cost-effective according to a recent cost-utility analysis.17 The model yielded an incremental cost-effectiveness ratio of $10,440 per quality-adjusted life-year saved as long as subsequent surveillance was limited to patients with Barrett's esophagus and dysplasia.17 This estimate was sensitive to the rate of progression to esophageal adenocarcinoma, but would hold true based on the annual risk of progression to esophageal adenocarcinoma of 0.4 percent and rates of malignant transformation in high-grade dysplasia found in the Danish cohort study described above.11 In addition, newer ultrathin endoscopy, which can be performed in physicians' offices without sedation, has the potential to significantly decrease the cost of screening and is well tolerated by patients.18
Although screening and surveillance for Barrett's esophagus is controversial, the latest evidence supports this approach to decrease morbidity and mortality. Carefully evaluating patients with reflux symptoms and stratifying them based on known risk factors (Table 15–10 ) has the potential to result in decreased cases of fatal esophageal adenocarcinoma in the United States.
