
Am Fam Physician. 2011;83(10):1211-1215
Related letter: Paroxetine Use Should Be Avoided During Pregnancy
Author disclosure: Nothing to disclose.
Clinical Question
Which antidepressants are safe to use during pregnancy?
Evidence-Based Answer
There are no studies that have shown any antidepressant to be absolutely safe for use during any stage of pregnancy. The use of selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs) during pregnancy does not increase the risk of congenital malformations or miscarriage. (Strength of Recommendation [SOR]: B, based on limited-quality, patient-oriented evidence.) The use of SSRIs or TCAs during pregnancy may increase the risk of preterm birth, low birth weight, respiratory distress, and neonatal convulsions, without obvious subsequent adverse neurodevelopmental outcomes. (SOR: B, based on limited-quality, patient-oriented evidence.) Table 11–8 lists reported outcomes and corresponding risks of antidepressant use during pregnancy.
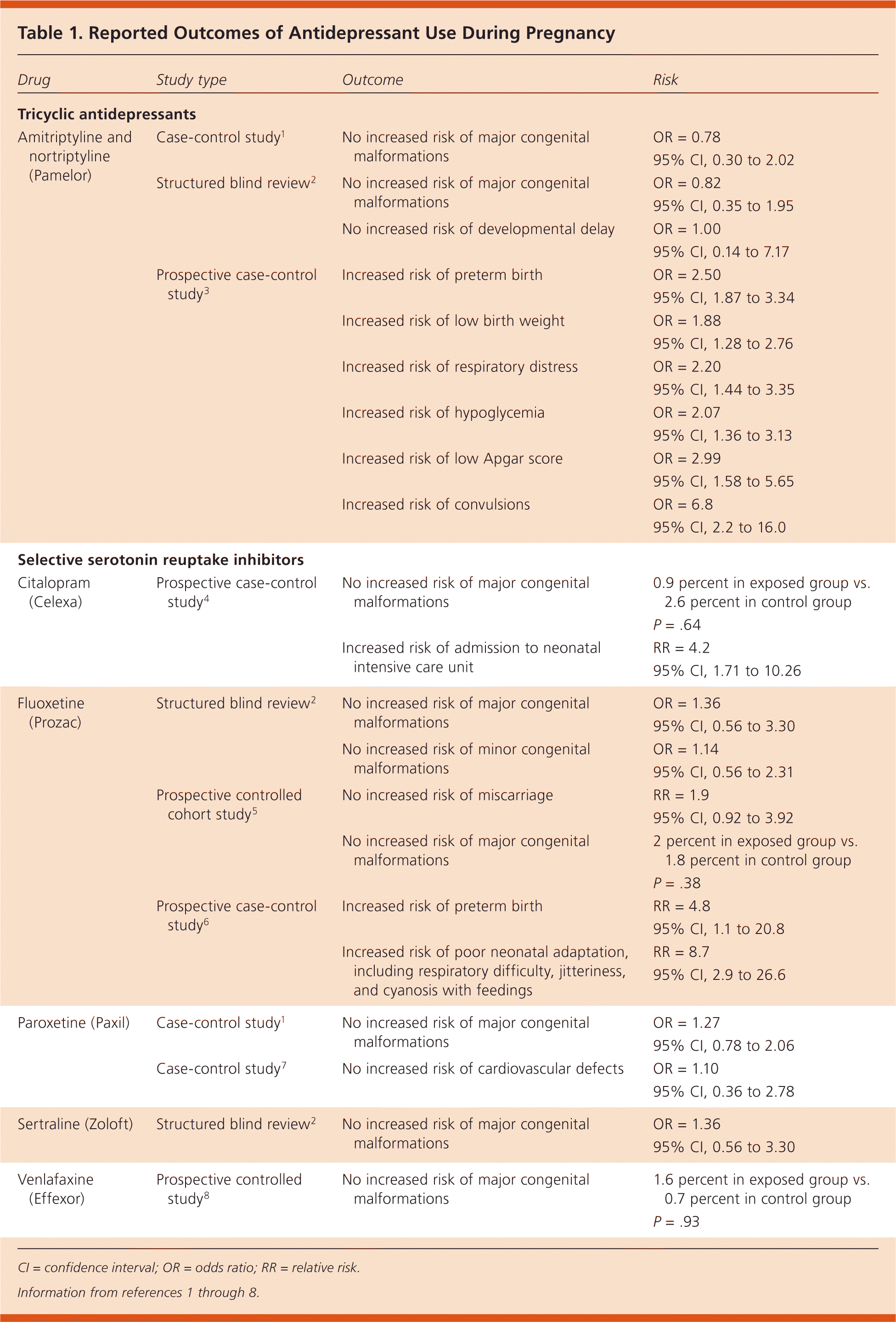
| Drug | Study type | Outcome | Risk |
|---|---|---|---|
| Tricyclic antidepressants | |||
| Amitriptyline and nortriptyline (Pamelor) | Case-control study1 | No increased risk of major congenital malformations | OR = 0.78 |
| 95% CI, 0.30 to 2.02 | |||
| Structured blind review2 | No increased risk of major congenital malformations | OR = 0.82 | |
| 95% CI, 0.35 to 1.95 | |||
| No increased risk of developmental delay | OR = 1.00 | ||
| 95% CI, 0.14 to 7.17 | |||
| Prospective case-control study3 | Increased risk of preterm birth | OR = 2.50 | |
| 95% CI, 1.87 to 3.34 | |||
| Increased risk of low birth weight | OR = 1.88 | ||
| 95% CI, 1.28 to 2.76 | |||
| Increased risk of respiratory distress | OR = 2.20 | ||
| 95% CI, 1.44 to 3.35 | |||
| Increased risk of hypoglycemia | OR = 2.07 | ||
| 95% CI, 1.36 to 3.13 | |||
| Increased risk of low Apgar score | OR = 2.99 | ||
| 95% CI, 1.58 to 5.65 | |||
| Increased risk of convulsions | OR = 6.8 | ||
| 95% CI, 2.2 to 16.0 | |||
| Selective serotonin reuptake inhibitors | |||
| Citalopram (Celexa) | Prospective case-control study4 | No increased risk of major congenital malformations | 0.9 percent in exposed group vs. 2.6 percent in control group |
| P = .64 | |||
| Increased risk of admission to neonatal intensive care unit | RR = 4.2 | ||
| 95% CI, 1.71 to 10.26 | |||
| Fluoxetine (Prozac) | Structured blind review2 | No increased risk of major congenital malformations | OR = 1.36 |
| 95% CI, 0.56 to 3.30 | |||
| No increased risk of minor congenital malformations | OR = 1.14 | ||
| 95% CI, 0.56 to 2.31 | |||
| Prospective controlled cohort study5 | No increased risk of miscarriage | RR = 1.9 | |
| 95% CI, 0.92 to 3.92 | |||
| No increased risk of major congenital malformations | 2 percent in exposed group vs. 1.8 percent in control group | ||
| P = .38 | |||
| Prospective case-control study6 | Increased risk of preterm birth | RR = 4.8 | |
| 95% CI, 1.1 to 20.8 | |||
| Increased risk of poor neonatal adaptation, including respiratory difficulty, jitteriness, and cyanosis with feedings | RR = 8.7 | ||
| 95% CI, 2.9 to 26.6 | |||
| Paroxetine (Paxil) | Case-control study1 | No increased risk of major congenital malformations | OR = 1.27 |
| 95% CI, 0.78 to 2.06 | |||
| Case-control study 7 | No increased risk of cardiovascular defects | OR = 1.10 | |
| 95% CI, 0.36 to 2.78 | |||
| Sertraline (Zoloft) | Structured blind review2 | No increased risk of major congenital malformations | OR = 1.36 |
| 95% CI, 0.56 to 3.30 | |||
| Venlafaxine (Effexor) | Prospective controlled study8 | No increased risk of major congenital malformations | 1.6 percent in exposed group vs. 0.7 percent in control group |
| P = .93 | |||
Evidence Summary
Data on antidepressant use during pregnancy are limited to retrospective studies and medication registries because of a lack of randomized controlled trials.
TRICYCLIC ANTIDEPRESSANTS
A case-control study found that TCA use during the first trimester was not associated with an increased risk of major congenital malformations.1
A structured blind review of 209 medical records also found no association between TCA use and major congenital malformations or developmental delay.2 A study using a birth registry that included 395 infants exposed to TCAs found an increased risk of preterm birth, low birth weight, respiratory distress, hypoglycemia, low Apgar score, and convulsions.3 A prospective study of 80 mothers taking TCAs found that in utero exposure does not affect global IQ, language development, or behavioral development in children 16 to 86 months of age.9
SELECTIVE SEROTONIN REUPTAKE INHIBITORS
Citalopram. A prospective case-control study of 125 women taking citalopram (Celexa) in the first trimester showed no association between citalopram use and major congenital malformations; however, use later in pregnancy is associated with an increased risk of admission to the neonatal intensive care unit.4
Fluoxetine. A prospective controlled cohort study of 128 pregnant women taking fluoxetine (Prozac) found no increased risk of major congenital malformations or difference in rates of miscarriage.5 A structured blind review of medical records of 185 pregnant women taking SSRIs, including 129 taking fluoxetine, showed no difference in rates of major or minor congenital malformations, developmental delay, or other neurologic disorders.2 A prospective case-control study found that third-trimester exposure to fluoxetine was associated with an increased risk of preterm birth, as well as poor neonatal adaptation, including respiratory difficulty, jitteriness, and cyanosis with feedings.6
Paroxetine. A case-control study using a medication and pregnancy registry that included 2,329 women found that first-trimester use of paroxetine (Paxil) was not associated with an increased risk of major congenital malformations.1 A case-control study of more than 3,000 infants with documented exposure to paroxetine during the first trimester found that rates of cardiovascular defects were 0.7 percent both in the group exposed to paroxetine and in the unexposed group.7
Sertraline. A structured blind review of medical records of 185 infants exposed to SSRIs, including 32 exposed to sertraline (Zoloft), showed no difference in rates of major congenital malformations or developmental delay.2
Venlafaxine. A prospective controlled study of 150 pregnant women taking venlafaxine (Effexor) in the first trimester concluded that the use of venlafaxine does not increase the risk of major congenital malformations.8
Little information is available on the use of duloxetine (Cymbalta), escitalopram (Lexapro), or bupropion (Wellbutrin) during pregnancy.
Recommendations from Others
The American College of Obstetricians and Gynecologists (ACOG) recommends avoiding paroxetine use during pregnancy. Fetal echocardiography should be considered if a woman takes paroxetine in early pregnancy. ACOG also recommends the use of a single medication at higher dosages over the use of multiple psychotropic medications. Treatment with SSRIs, selective norepinephrine reuptake inhibitors, or both should be individualized.10
