
Am Fam Physician. 2011;83(12):1432-1437
A more recent article on herpes zoster and postherpetic neuralgia is available.
Patient information: See related handout on shingles, written by the authors of this article.
Author disclosure: Nothing to disclose.
Herpes zoster (shingles) is diagnosed clinically by recognition of the distinctive, painful vesicular rash appearing in a unilateral, dermatomal distribution. An estimated 1 million cases occur in the United States each year, and increasing age is the primary risk factor. Laboratory testing, including polymerase chain reaction, can confirm atypical cases. Treatment with acyclovir, famciclovir, or valacyclovir decreases the duration of the rash. Adjunct medications, including opioid analgesics, tricyclic antidepressants, or corticosteroids, may relieve the pain associated with acute herpes zoster. There is conflicting evidence that antiviral therapy during the acute phase prevents postherpetic neuralgia. Postherpetic neuralgia in the cutaneous nerve distribution may last from 30 days to more than six months after the lesions have healed. Evidence supports treating postherpetic neuralgia with tricyclic antidepressants, gabapentin, pregabalin, long-acting opioids, or tramadol; moderate evidence supports the use of capsaicin cream or a lidocaine patch as a second-line agent. Immunization to prevent herpes zoster and postherpetic neuralgia is recommended for most adults 60 years and older.
Herpes zoster (shingles) presents as a painful vesicular rash and is caused by reactivation of the varicella-zoster virus within the dorsal root or cranial nerve ganglia. Approximately 1 million cases occur in the United States each year,1 with an incidence of 3.2 cases per 1,000 person-years.2 Increasing age is the primary risk factor for herpes zoster. The disease usually occurs between 50 and 79 years of age, and approximately 60 percent of cases occur in women.2 Other risk factors include human immunodeficiency virus infection, neoplastic diseases, organ transplantation, use of immunosuppressive drugs, and other conditions that cause a decline in cell-mediated immunity.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Antiviral therapy should be initiated within 72 hours of onset of the rash in patients with acute herpes zoster to increase the rate of healing and decrease pain. | A | 10–12 |
| Based on individual patient characteristics, a tricyclic antidepressant, tramadol (Ultram), long-acting opioid, or anticonvulsant (i.e., gabapentin [Neurontin] or pregabalin [Lyrica]) should be selected to decrease the pain of postherpetic neuralgia. | A | 7, 19–21 |
| If topical therapy is indicated, capsaicin cream (Zostrix) or a lidocaine patch (Lidoderm) may decrease pain in patients with postherpetic neuralgia. | B | 7, 19, 20, 22 |
| Herpes zoster vaccine (Zostavax) should be given to most persons 60 years and older to prevent herpes zoster and postherpetic neuralgia. | B | 25, 27 |
Clinical Presentation
Prodromal symptoms include malaise, headache, photophobia, abnormal skin sensations, and occasionally fever. These symptoms may occur one to five days before the appearance of the rash.4 Abnormal skin sensations range from itching and burning to hyperesthesia and severe pain. The intensity of the pain may lead to a misdiagnosis, such as renal colic or myocardial infarction, depending on location.
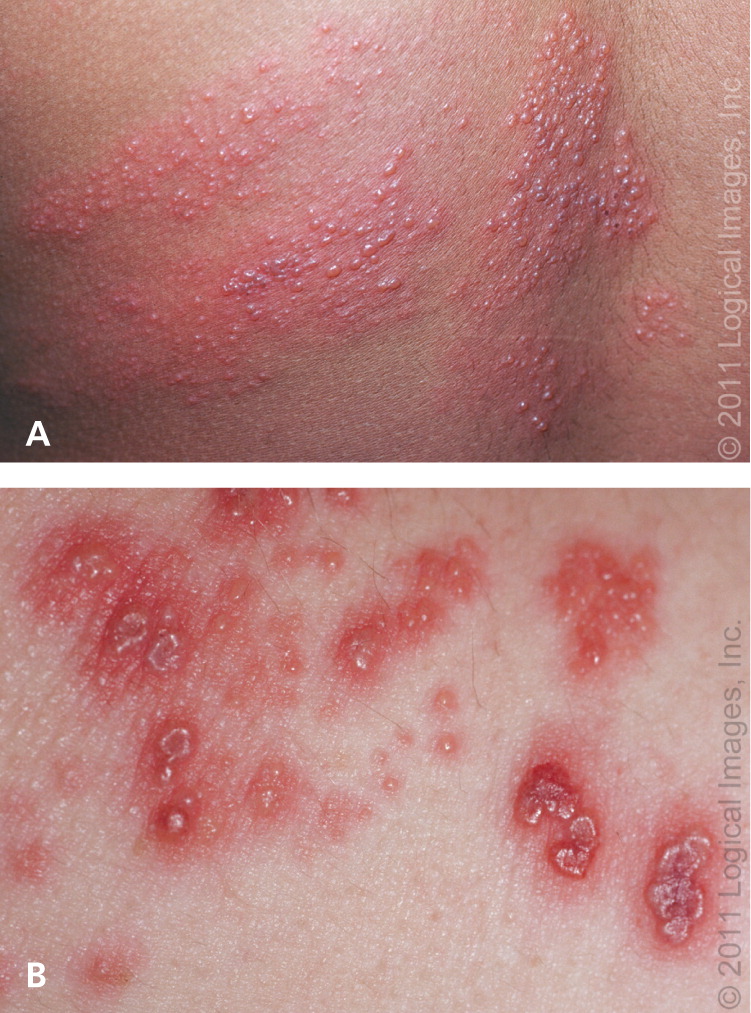
The rash (Figure 1) begins as maculopapular lesions in a unilateral, dermatomal distribution that rarely crosses the midline. Lesions progress to clear vesicles that become cloudy within three to five days, then crust and heal within two to four weeks. Scarring and changes in pigmentation may occur. Dermatomes of the back and face are most often affected, although multiple dermatome involvement is possible.
Diagnosis
The diagnosis of herpes zoster is usually clinical, based on recognition of the distinctive presentation and rash. Cases of herpes zoster without a rash (zoster sine herpete) are difficult to diagnose and require laboratory confirmation of varicella-zoster virus reactivation.5 Fluid obtained from vesicles may be evaluated with polymerase chain reaction testing, viral culture, or direct immunofluorescent antigen staining.6 Table 1 lists the accuracy of diagnostic testing for herpes zoster.6
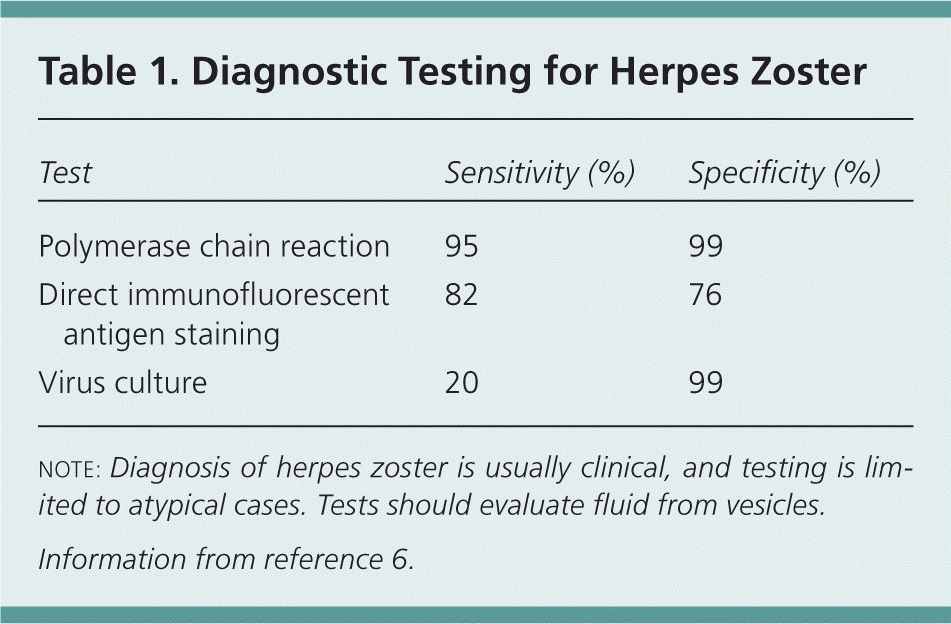
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Polymerase chain reaction | 95 | 99 |
| Direct immunofluorescent antigen staining | 82 | 76 |
| Virus culture | 20 | 99 |
Complications
Postherpetic neuralgia is the most common complication of herpes zoster. It occurs in approximately 30 percent of patients older than 80 years and in approximately 20 percent of patients 60 to 65 years; it is rare in patients younger than 50 years.7 Replication of the varicella-zoster virus in the basal ganglia destroys the nerves, leading to pain in the affected dermatome. Postherpetic pain may take several forms, including allodynia (nonpainful stimulus perceived as painful), hyperpathia (slightly painful stimulus perceived as very painful), and dysesthesia (abnormal sensation with no stimuli).8
Women are at greater risk of postherpetic neuralgia. Additional risk factors include older age, moderate to severe rash, moderate to severe acute pain during the rash, ophthalmic involvement, and history of prodromal pain.8,9 Postherpetic neuralgia may persist from 30 days to more than six months after the lesions have healed, and most cases resolve spontaneously.7
Herpes zoster ophthalmicus (ophthalmic zoster) occurs in 5 to 10 percent of patients with herpes zoster and may lead to permanent vision loss and cranial nerve palsies.8 Urgent ophthalmology referral is recommended in these patients. Superimposed bacterial skin infections with streptococci and staphylococci should be treated with appropriate oral antibiotics. Encephalitis, meningitis, myelitis, and disseminated cutaneous and visceral disease may occur in patients with severe immunosuppression.
Management of Acute Herpes Zoster
Antiviral therapy is first-line treatment and should be initiated within 72 hours of rash onset to increase the rate of healing and decrease pain.10–12 No study has investigated the effectiveness of later initiation of antiviral therapy, but it is believed to benefit patients with active vesicle eruptions.3
Acyclovir (Zovirax), famciclovir (Famvir), and valacyclovir (Valtrex) are approved by the U.S. Food and Drug Administration (FDA) for the treatment of acute herpes zoster. These agents are considered safe and are well tolerated with minimal adverse effects (e.g., headache, nausea). All three drugs are available in a generic form, although acyclovir is significantly less expensive than famciclovir or valacyclovir 13 (Table 2). These antiviral agents decrease the severity and duration of acute herpes zoster. In one randomized controlled trial, valacyclovir led to complete pain resolution sooner than acyclovir (44 versus 51 days) and required less frequent dosing.14 Famciclovir has shown similar effectiveness to valacyclovir.10 Topical antiviral agents are not effective.3
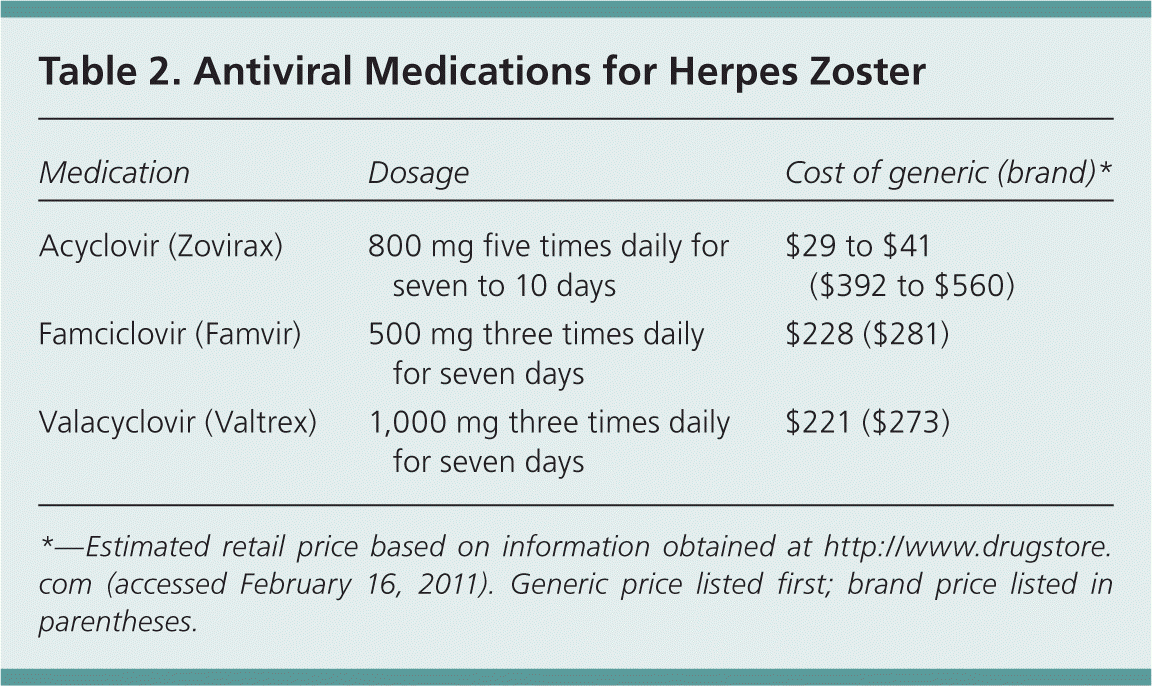
| Medication | Dosage | Cost of generic (brand)* |
|---|---|---|
| Acyclovir (Zovirax) | 800 mg five times daily for seven to 10 days | $29 to $41 ($392 to $560) |
| Famciclovir (Famvir) | 500 mg three times daily for seven days | $228 ($281) |
| Valacyclovir (Valtrex) | 1,000 mg three times daily for seven days | $221 ($273) |
Although antiviral medications slow the production of the virus and decrease the viral load in the dorsal root ganglia, evidence showing that these medications alter the incidence and course of postherpetic neuralgia is inconsistent. Some studies suggest that no antiviral agent prevents postherpetic neuralgia,8,15 whereas others report reduced duration of symptoms.4,7,16 Famciclovir and valacyclovir are associated with better outcomes than placebo or acyclovir.11,14
Antivirals alone are usually insufficient to relieve the often debilitating pain of acute herpes zoster. Mild to moderate pain may be controlled with acetaminophen or nonsteroidal anti-inflammatory drugs, alone or in combination with a weak opioid or tramadol (Ultram).3 Moderate to severe pain requires scheduled opioids (e.g., oxycodone, morphine).3 The intensity of pain during the acute attack is an important predictor for the development of postherpetic neuralgia, and medications given during this phase may influence the outcome of later interventions for postherpetic neuralgia.17
If pain does not rapidly respond to opioid analgesics or if opioids are not tolerated, the prompt addition of an adjunctive therapy should be considered.3 Nortriptyline (Pamelor), gabapentin (Neurontin), and pregabalin (Lyrica) have been recommended, but they have not been extensively studied for pain relief in patients with acute herpes zoster.3
The addition of corticosteroids to acyclovir decreases the pain of acute herpes zoster 17 and speeds lesion healing and return to daily activities. Combination therapy with corticosteroids and antivirals should be considered in older patients with no contraindications.17 Although corticosteroids have anti-inflammatory effects that could be expected to decrease nerve damage and the risk of postherpetic neuralgia, a Cochrane review found no significant difference between corticosteroids and placebo in preventing postherpetic neuralgia six months after onset of the rash.18
Management of Postherpetic Neuralgia
Theoretical models suggest that reducing pain during the acute phase of herpes zoster may stop the initiation of the mechanisms that cause chronic pain, thus reducing the risk of postherpetic neuralgia.4 If the condition develops, treatment focuses on preventing a chronic pain syndrome. Several medications have proved effective for postherpetic neuralgia (Table 319 ) and should be selected based on individual patient characteristics.7,19–22 Many of these medications require dosing adjustment in older patients and in those with reduced creatinine clearance.
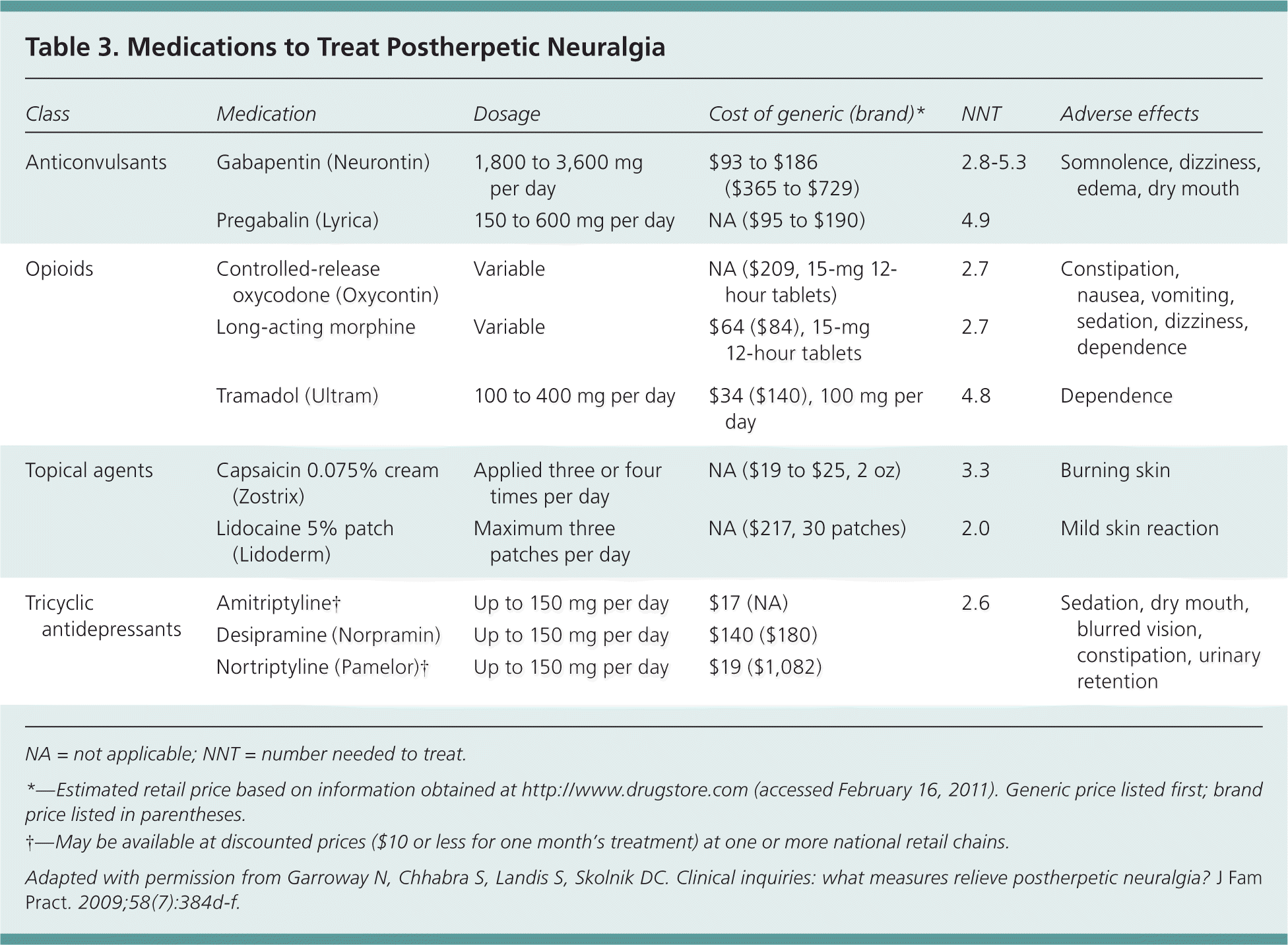
| Class | Medication | Dosage | Cost of generic (brand)* | NNT | Adverse effects |
|---|---|---|---|---|---|
| Anticonvulsants | Gabapentin (Neurontin) | 1,800 to 3,600 mg per day | $93 to $186 ($365 to $729) | 2.8–5.3 | Somnolence, dizziness, edema, dry mouth |
| Pregabalin (Lyrica) | 150 to 600 mg per day | NA ($95 to $190) | 4.9 | ||
| Opioids | Controlled-release oxycodone (Oxycontin) | Variable | NA ($209, 15-mg 12-hour tablets) | 2.7 | Constipation, nausea, vomiting, sedation, dizziness, dependence |
| Long-acting morphine | Variable | $64 ($84), 15-mg 12-hour tablets | 2.7 | ||
| Tramadol (Ultram) | 100 to 400 mg per day | $34 ($140), 100 mg per day | 4.8 | Dependence | |
| Topical agents | Capsaicin 0.075% cream (Zostrix) | Applied three or four times per day | NA ($19 to $25, 2 oz) | 3.3 | Burning skin |
| Lidocaine 5% patch (Lidoderm) | Maximum three patches per day | NA ($217, 30 patches) | 2.0 | Mild skin reaction | |
| Tricyclic antidepressants | Amitriptyline† | Up to 150 mg per day | $17 (NA) | 2.6 | Sedation, dry mouth, blurred vision, constipation, urinary retention |
| Desipramine (Norpramin) | Up to 150 mg per day | $140 ($180) | |||
| Nortriptyline (Pamelor) | Up to 150 mg per day | $19 ($1,082) |
Tricyclic antidepressants, such as amitriptyline, desipramine (Norpramin), and nortriptyline, have pain-modulating effects in neuropathic and other chronic pain states. They are the mainstay of treatment for postherpetic neuralgia, and evidence supports their effectiveness.20 In older patients, tricyclic antidepressants should be started at lower doses given at bedtime, and the patient should be monitored for adverse effects, including interactions with other medications.
Opioid medications have analgesic effects and are helpful for postherpetic neuralgia. Studies have shown that patients taking oxycodone, morphine, or methadone have better pain relief than those taking placebo.7 Opioids should be carefully adjusted in all patients for clinical response. Oxycodone is of special concern because of a 50 percent higher serum concentration when creatinine clearance is less than 60 mL per minute per 1.73 m2 (1.00 mL per second per m2).23 Morphine and methadone have been shown to provide better pain relief than tricyclic antidepressants.7,20 A Cochrane review of seven trials found tramadol to be effective for neuropathic pain, including postherpetic neuralgia.21
Studies involving anticonvulsants showed that gabapentin and pregabalin reduce pain from postherpetic neuralgia by approximately 50 percent.7,19,20 Another study comparing the maximum tolerated dosages of gabapentin and nortriptyline found that both reduced pain scores, but gabapentin was associated with fewer adverse effects.7
The FDA has approved two topical medications for treatment of postherpetic neuralgia. A Cochrane review that included a few small studies of topical lidocaine patches (Lidoderm) reported benefit in some patients, but found insufficient evidence to recommend them as first-line therapy.22 Based on poor-quality studies, capsaicin cream (Zostrix), which is derived from peppers, provides limited reduction in postherpetic neuralgia.7,19,20 Topical antiviral agents, such as idoxuridine (not available in the United States), may reduce the prevalence of postherpetic neuralgia in immunocompetent patients, but the evidence is low-quality.7 Intrathecal preservative-free methylprednisolone (not available in the United States) is effective for intractable postherpetic neuralgia. However, it should be used only if other agents have been ineffective.20
Other treatments for postherpetic neuralgia have been investigated, but not all are effective. Aspirin cream has been helpful in a few small studies, but the benefit is not considered significant.20 No recommendations can be made for the anticonvulsants valproate (Depakote)19 and carbamazepine (Tegretol)20 because of limited data. Anesthetic agents such as N-methyl-d-aspartate receptor antagonists play a role in processing pain signals and could potentially benefit patients with postherpetic neuralgia. Ketamine (Ketalar), dextromethorphan, and memantine (Namenda) have not been shown to improve pain compared with placebo.20
Prevention
Herpes zoster and postherpetic neuralgia are preventable conditions. The Centers for Disease Control and Prevention recommends one dose of the herpes zoster vaccine (Zostavax) for persons 60 years and older.24,25 The FDA recently approved the herpes zoster vaccine for healthy patients between 50 and 59 years of age, and the Advisory Committee on Immunization Practices is expected to vote on recommending the vaccine for this population in late 2011.26 Patients may be immunized without serologic testing and regardless of any history of varicella virus infection or herpes zoster.24,25 Coadministration with other inactivated vaccines common in this age group is considered safe.24 Special considerations for herpes zoster vaccination are presented in Table 4.9,24,26
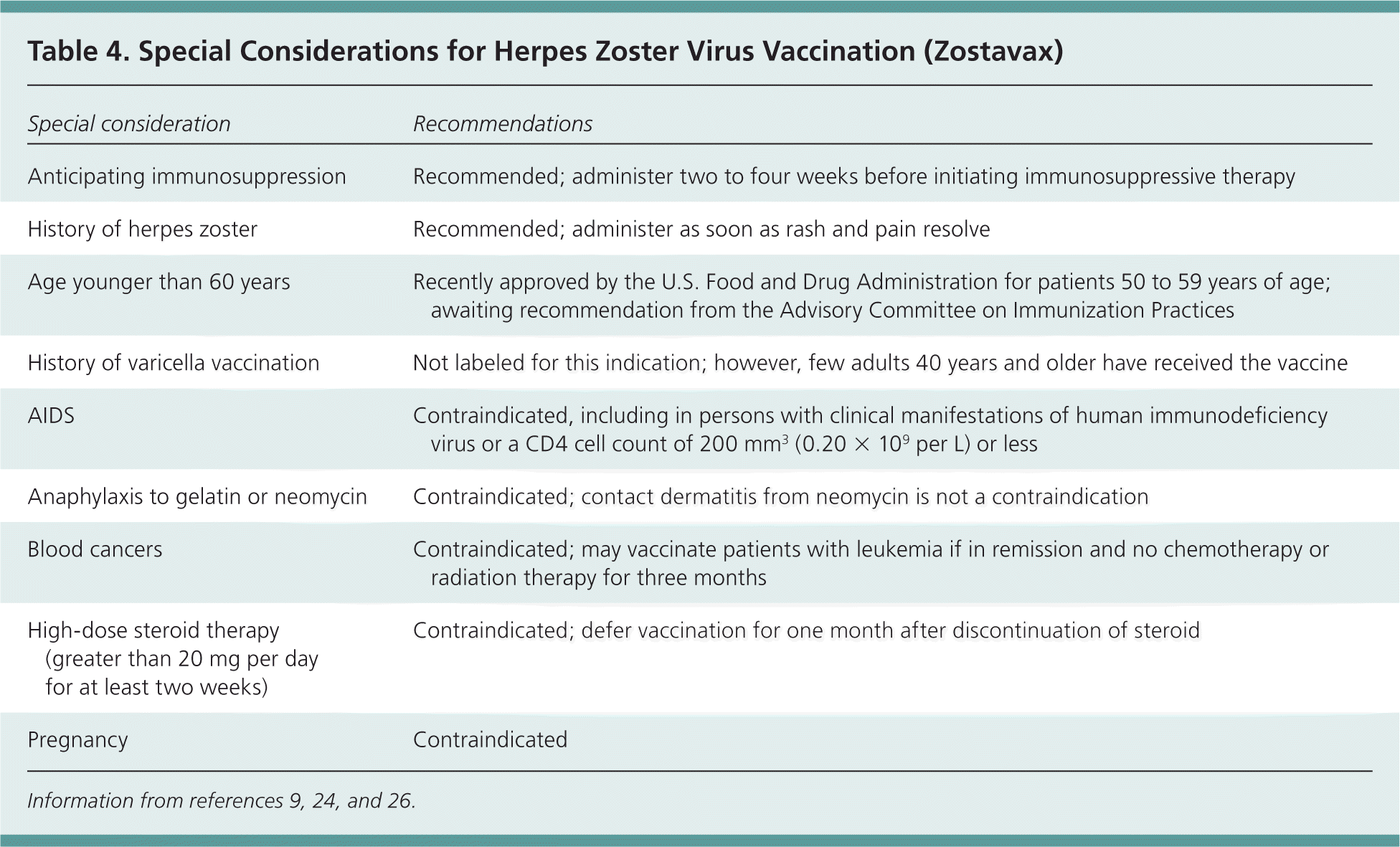
| Special consideration | Recommendations |
|---|---|
| Anticipating immunosuppression | Recommended; administer two to four weeks before initiating immunosuppressive therapy |
| History of herpes zoster | Recommended; administer as soon as rash and pain resolve |
| Age younger than 60 years | Recently approved by the U.S. Food and Drug Administration for patients 50 to 59 years of age; awaiting recommendation from the Advisory Committee on Immunization Practices |
| History of varicella vaccination | Not labeled for this indication; however, few adults 40 years and older have received the vaccine |
| AIDS | Contraindicated, including in persons with clinical manifestations of human immunodeficiency virus or a CD4 cell count of 200 mm3 (0.20 × 109 per L) or less |
| Anaphylaxis to gelatin or neomycin | Contraindicated; contact dermatitis from neomycin is not a contraindication |
| Blood cancers | Contraindicated; may vaccinate patients with leukemia if in remission and no chemotherapy or radiation therapy for three months |
| High-dose steroid therapy (greater than 20 mg per day for at least two weeks) | Contraindicated; defer vaccination for one month after discontinuation of steroid |
| Pregnancy | Contraindicated |
The Shingles Prevention Study found the herpes zoster vaccine to be 51.3 percent effective in preventing herpes zoster and 66.5 percent effective in preventing postherpetic neuralgia (when defined as pain rated as at least a 3 out of 10 on a severity scale that persisted for at least 90 days after rash onset).27 Vaccination has also been shown to reduce the incidence of postherpetic neuralgia by 39 percent among patients who develop herpes zoster.24 The number needed to treat to prevent one case over three years is 58 for herpes zoster and 364 for postherpetic neuralgia.9
Fewer than 10 percent of eligible persons receive the herpes zoster vaccine.28 Patient surveys show that many do not know about it or believe it is not needed.29 Cost is a significant issue with the vaccine. The average wholesale cost of Zostavax is $194 per dose,13 and many insurance plans do not cover it. Another concern is storage. The vaccine must be frozen, and in-office administration requires a monitored, temperature-controlled freezer. Patients may be referred to a pharmacy for immunization or given a prescription for the vaccine, which must be kept cold and administered within 30 minutes.8 Physicians can play a key role in overcoming these barriers and should encourage vaccination to prevent herpes zoster and postherpetic neuralgia.
Data Sources: We received two evidence summaries from AFP. The first, PubMed “clinical queries search for herpes+zoster,” yielded meta-analyses and systematic reviews. The second, “evidence report for herpes zoster,” included InfoPOEMs, Cochrane reviews, and practice guidelines. We also used primeanswers.org to search PubMed for postherpetic neuralgia within ACP Journal Club, Clinical Evidence, Cochrane Database of Systematic Reviews, FPIN Clinical Inquiries, NEJM Clinical Practice, and U.S. Preventive Services Task Force. In addition, we searched Dynamed for herpes zoster and postherpetic neuralgia. Search dates: AFP's searches, May 10, 2010; our searches, May 12, 2010.
