
Am Fam Physician. 2011;84(4):427-434
Related letter: Vitamin C for Preventing Exercise-Induced Asthma
Patient information: See related handout on exercise-induced wheezing, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Exercise-induced bronchoconstriction describes the narrowing of the airway that occurs with exercise. More than 10 percent of the general population and up to 90 percent of persons previously diagnosed with asthma have exercise-induced bronchoconstriction. Common symptoms include coughing, wheezing, and chest tightness with exercise; however, many athletes will present with nonspecific symptoms, such as fatigue and impaired performance. Spirometry should be performed initially to evaluate for underlying chronic asthma, although results are often normal. An empiric trial of short-acting beta2 agonists or additional bronchial provocation testing may be necessary to confirm the diagnosis. Nonpharmacologic treatment options include avoiding known triggers, choosing sports with low minute ventilation, warming up before exercising, and wearing a heat exchange mask in cold weather. Short-acting beta2 agonists are recommended first-line agents for pharmacologic treatment, although leukotriene receptor antagonists or inhaled corticosteroids with or without long-acting beta2 agonists may be needed in refractory cases. If symptoms persist despite treatment, alternative diagnoses such as cardiac or other pulmonary etiologies, vocal cord dysfunction, or anxiety should be considered.
Exercise-induced bronchoconstriction (EIB) is a common problem in physically active persons. EIB is defined as transient, reversible bronchoconstriction that happens during or after strenuous exercise. It can occur in persons with or without underlying asthma.1 Bronchoconstriction that is triggered by exercise in persons with underlying asthma is considered exercise-induced asthma. This article discusses the diagnosis and management of EIB in persons without underlying asthma. The National Asthma Education and Prevention Program also provides guidelines for the diagnosis and management of asthma.2
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Self-reported symptoms alone should not be used to diagnose EIB. | C | 6–8 | — |
| The exercise challenge test can accurately diagnose EIB. | C | 9 | Varying results because of differences in how the test is performed |
| Warming up before exercise may reduce the degree of EIB. | B | 2, 14 | — |
| Wearing a heat exchange mask over the mouth and nose during cold-weather exercise may reduce symptoms of EIB. | B | 2, 16 | — |
| Inhaled short-acting beta2 agonist use before exercise can attenuate symptoms of EIB. | A | 2, 14, 21 | — |
| Using inhaled corticosteroids as controller therapy is an effective management strategy for EIB in patients with underlying asthma. | A | 2, 14, 25 | — |
| Leukotriene receptor antagonist therapy can effectively manage EIB. | A | 14, 26, 29 | — |
EIB = exercise-induced bronchoconstriction.
A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, go to https://www.aafp.org/afpsort.xml.
Epidemiology and Etiology
EIB is more common in persons who participate in endurance sports and sports that require high minute ventilation. More than 10 percent of the general population and up to 90 percent of persons previously diagnosed with asthma have EIB.3 The prevalence of EIB in athletes ranges from 11 to 50 percent, although it approaches 90 percent in athletes with asthma.3 EIB also commonly occurs in cold-weather athletes and has been found to be present in approximately 50 percent of Olympic cross-country skiers.4
In persons without asthma, the rapid breathing of cold, dry air over a prolonged period is an ideal setting for EIB. When a person finishes exercising, the airway responds with vasodilation to warm the airway, resulting in water loss and engorgement of the airways. This process causes bronchoconstriction and the release of proinflammatory mediators. Another possible etiology may be environmental irritants, such as chlorine gas in a swimming pool or gases from ice-resurfacing equipment. Persons with EIB and underlying asthma usually experience exacerbation of underlying inflammation and airway hyperactivity caused by any of these mechanisms or by poorly controlled chronic asthma.5
Diagnosis
SYMPTOMS
Typical symptoms of EIB include wheezing, shortness of breath, dyspnea, cough, or chest tightness during or after exercise. These symptoms usually occur during strenuous exercise and peak about five to 10 minutes after exercise. Atypical symptoms include fatigue, feeling out of shape, feeling unable to keep up with peers, and abdominal discomfort. Self-reported symptoms have been shown to be poor predictors of EIB because other conditions, such as vocal cord dysfunction, can cause similar symptoms.6–8 Therefore, symptoms alone should not be used to diagnose EIB.
The physical examination in patients with EIB is often unremarkable. If patients are evaluated when symptomatic, the most common findings are tachypnea and wheezing during end expiration.
TESTING
In patients with possible EIB, spirometry should be performed to rule out underlying asthma2 (Figure 1). Normal resting spirometry results are common in patients with EIB. If spirometry reveals an obstruction, additional testing before and after albuterol use is recommended. Obstruction with reversibility is indicative of underlying chronic persistent asthma.
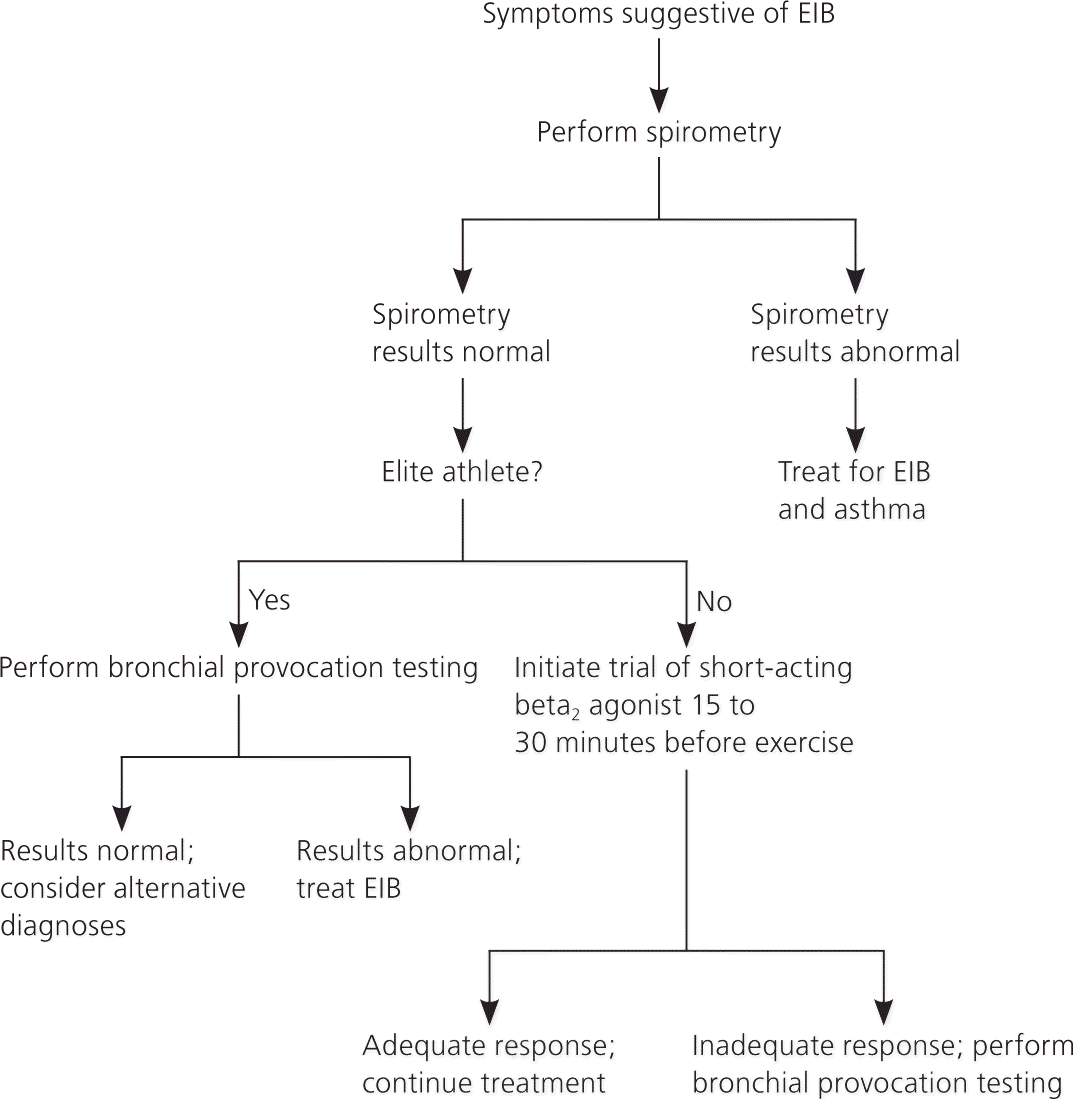
If the patient is not an elite athlete, the next step is to prescribe an empiric trial of a short-acting beta2 agonist. Only athletes who participate in high-level competition will need documentation of objective testing to use banned asthma medication. Follow-up, usually after one to two weeks, is necessary to determine whether treatment is successful. If the patient's response to treatment is inadequate, additional testing is warranted.
For elite athletes or for persons with EIB that does not respond to a trial of a short-acting beta2 agonist, bronchial provocation tests can be used to identify provoked decreases in forced expiratory volume in one second (FEV1). There are two main types of bronchial provocation tests: direct and indirect. Most pulmonary function laboratories can perform direct challenges, such as methacholine challenge. Some are also equipped to perform indirect challenges, such as an exercise challenge test, which can accurately diagnose EIB.9 In addition, indirect challenges can be performed with a handheld spirometer at the location in which the athlete develops symptoms. Direct challenges have a lower sensitivity than indirect challenges10; therefore, indirect challenge is the preferred diagnostic test for EIB. Examples of indirect testing are listed in Table 1.9 If bronchial provocation testing results are normal, other diagnoses should be considered (Table 211 ).
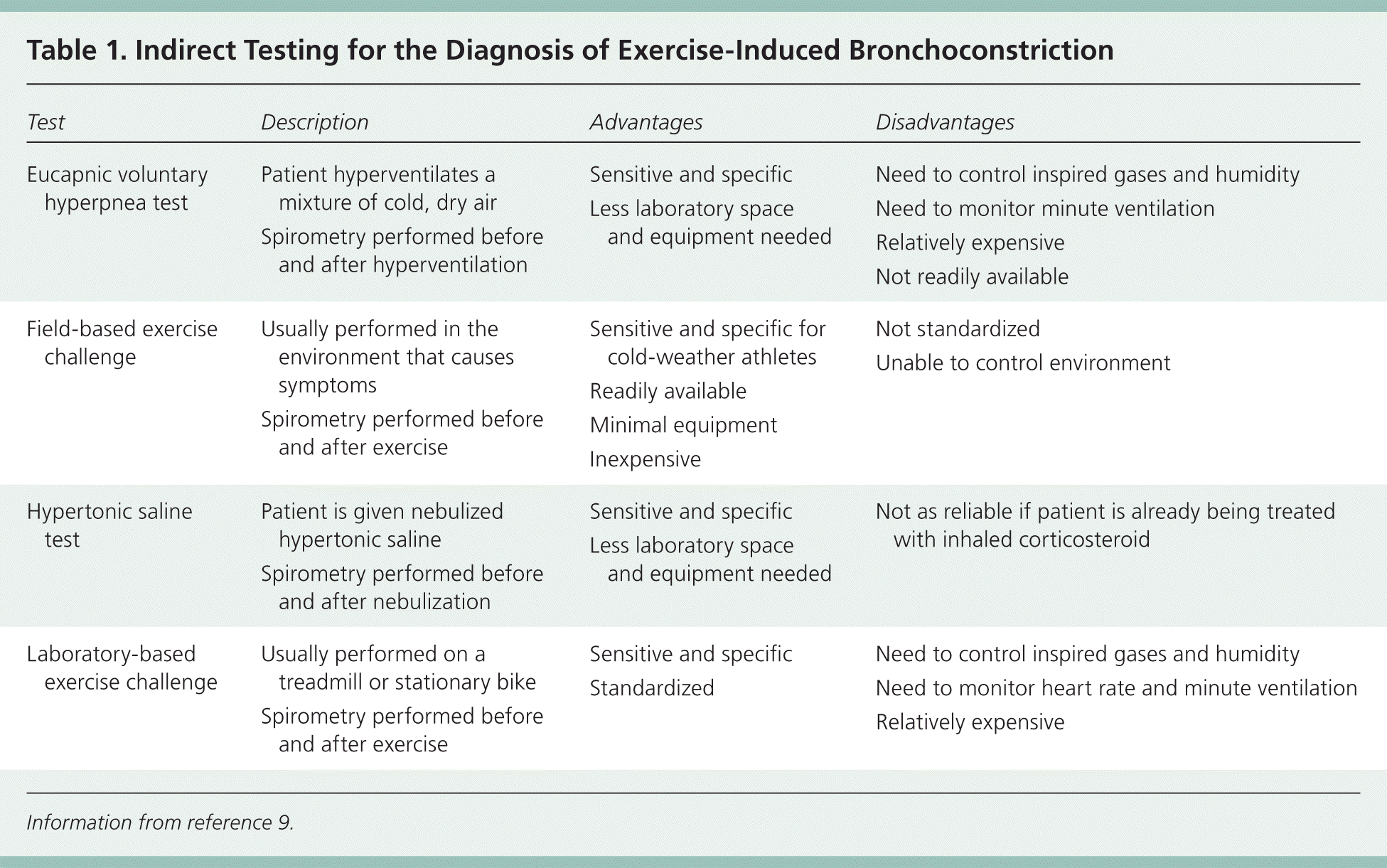
| Test | Description | Advantages | Disadvantages |
|---|---|---|---|
| Eucapnic voluntary hyperpnea test | Patient hyperventilates a mixture of cold, dry air |
|
|
| Spirometry performed before and after hyperventilation | |||
| Field-based exercise challenge | Usually performed in the environment that causes symptoms |
|
|
| Spirometry performed before and after exercise | |||
| Hypertonic saline test | Patient is given nebulized hypertonic saline |
|
|
| Spirometry performed before and after nebulization | |||
| Laboratory-based exercise challenge | Usually performed on a treadmill or stationary bike |
|
|
| Spirometry performed before and after exercise |
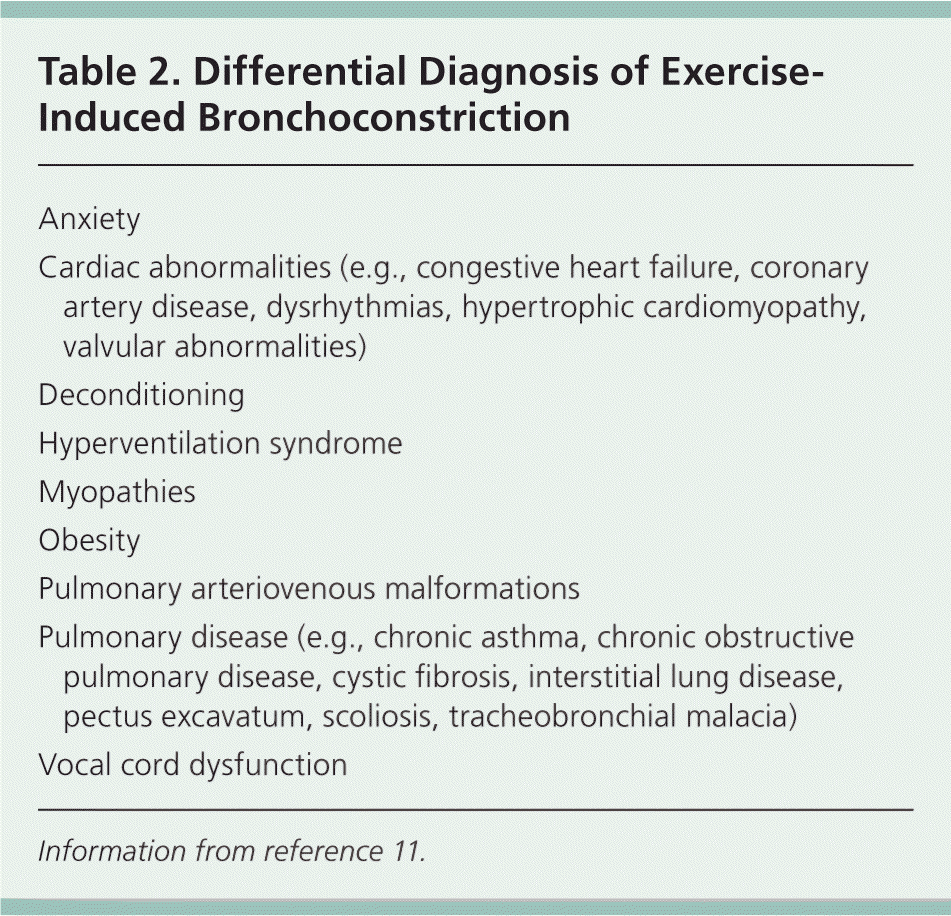
| Anxiety |
| Cardiac abnormalities (e.g., congestive heart failure, coronary artery disease, dysrhythmias, hypertrophic cardiomyopathy, valvular abnormalities) |
| Deconditioning |
| Hyperventilation syndrome |
| Myopathies |
| Obesity |
| Pulmonary arteriovenous malformations |
| Pulmonary disease (e.g., chronic asthma, chronic obstructive pulmonary disease, cystic fibrosis, interstitial lung disease, pectus excavatum, scoliosis, tracheobronchial malacia) |
| Vocal cord dysfunction |
Management
EIB can affect many aspects of a patient's life, regardless of the severity of symptoms. The main goal of treatment is to allow patients to exercise safely. Secondary goals should include keeping athletes of all levels active and helping competitive athletes maximize performance. Asthma symptoms in association with exercise have been shown to reduce health-related quality of life scores in adolescents.12 One report found that among asthma-related deaths during exercise, many athletes had only mild asthma.13
NONPHARMACOLOGIC TREATMENT
Several nonpharmacologic options exist for managing EIB. Basic measures include avoiding known triggers (allergen and environmental) and choosing sports with low minute ventilation (short bursts of exercise), such as football, baseball, wrestling, or sprinting. Although nonpharmacologic treatment options can be effective, all athletes with EIB need to have a short-acting beta2 agonist available.
Heat Exchange Mask. Heat exchange masks are designed to limit cold air exposure during exercise in athletes with EIB. They typically can be found in running stores or online. Using a mask has not been shown to be as effective as pretreatment albuterol in the prevention of bronchoconstriction.2,16 One of the main limitations of a heat exchange mask is that it may not be practical during competition.
Nutrition. A review of the literature suggests that restricting dietary sodium intake for one to two weeks may reduce bronchoconstriction after exercise in patients with asthma and EIB; however, long-term studies are lacking.17 Additionally, a small, double-blind crossover study showed that high-dose omega-3 fish oil supplementation for three weeks reduced the use of bronchodilators in the treatment group. The limitations of this study included the small size and no mention of adverse effects from the high dosage of omega-3 fish oil.18
PHARMACOLOGIC TREATMENT
| Medication | Dosage | Onset of action | Duration | Comments | Cost of generic (brand)* | |
|---|---|---|---|---|---|---|
| Short-acting beta2agonists | ||||||
| Albuterol | 90 mcg per spray; two puffs 15 minutes before exercise and as needed | Five to seven minutes | Three to six hours | First-line prevention of acute asthma | 90 mcg per actuation, 17-g inhaler: $29 to $58 (NA) † | |
| Levalbuterol (Xopenex HFA) | 45 mcg per spray; two puffs 15 minutes before exercise and as needed | Five to 10 minutes | Three to six hours | First-line prevention of acute asthma; similar to albuterol in safety and effectiveness, but more expensive | Box of 24 | |
| 1.25 mg per 3 mL vials: NA ($123) | ||||||
| 0.63 mg per 3 mL vials: NA ($136) | ||||||
| 0.31 mg per 3 mL vials: NA ($121) | ||||||
| Pirbuterol (Maxair) | 200 mcg per spray; one or two puffs 15 minutes before exercise and as needed | Five minutes | Five hours | First-line prevention of acute asthma; not available as generic; more expensive than albuterol | 200 mcg per actuation, 14-g inhaler: NA ($146) | |
| Long-acting beta2agonists | ||||||
| Formoterol (Foradil Aerolizer); combination budesonide/formoterol (Symbicort) | Aerolizer: 12 mcg per capsule; one spray twice per day | One to three minutes | 12 hours | Second-line prevention of chronic asthma in conjunction with inhaled corticosteroid | Aerosol: 60 12-mcg capsules: NA ($176) | |
| Metered-dose inhaler: 4.5 mcg per spray; two puffs twice per day | Inhaler | |||||
| 80/4.5 mcg per actuation aerosol, 10.2-g inhaler: NA ($196) | ||||||
| 160/4.5 mcg per actuation aerosol, 10.2-g inhaler: NA ($230) | ||||||
| Salmeterol (Serevent Diskus); combination fluticasone/salmeterol (Advair Diskus or HFA) | Diskus: 50 mcg per blister; one puff twice per day | 30 to 48 minutes | 12 hours | Second-line prevention of chronic asthma in conjunction with inhaled corticosteroid | Serevent Diskus (60 doses) | |
| 50 mcg per dose powder inhaler: NA ($178) | ||||||
| Advair Diskus (60 doses) | ||||||
| 100/50 mcg per dose aerosol: NA ($186) | ||||||
| 250/50 mcg per dose aerosol: NA ($216) | ||||||
| 500/50 mcg per dose aerosol: NA ($286) | ||||||
| HFA: 21 mcg per puff; two puffs twice per day | Advair HFA | |||||
| 45/21 mcg per actuation aerosol, 12-g inhaler: NA ($198) | ||||||
| 115/21 mcg per actuation aerosol, 12-g inhaler: NA ($231) | ||||||
| 230/21 mcg per actuation aerosol, 12-g inhaler: NA ($296) | ||||||
| Mast cell stabilizers | ||||||
| Cromolyn (nebulizer solution) | 20 mg per 2 mL; 20 mg taken 10 to 60 minutes before exercise or 20 mg four times per day | Less than one week | Unknown | Second-line prevention of chronic asthma | 120 vials: 20 mg per 2 mL: $190 (NA) | |
| Inhaled corticosteroids | ||||||
| Beclomethasone (QVAR) | 40 or 80 mcg per spray; one to four puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 40 mcg per actuation, 8.7-g inhaler: NA ($110) | |
| 80 mcg per actuation, 8.7-g inhaler: NA ($132) | ||||||
| Budesonide (Pulmicort Flexhaler) | 90 or 180 mcg per spray; two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 90 mcg per actuation aerosol inhaler: NA ($122) | |
| 180 mcg per actuation aerosol inhaler: NA ($160) | ||||||
| Ciclesonide (Alvesco) | 80 or 160 mcg per spray; one or two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 80 mcg per actuation aerosol, 6.1-g inhaler: NA ($166) | |
| 160 mcg per actuation aerosol, 6.1-g inhaler: NA ($167) | ||||||
| Flunisolide | 250 mcg per spray; one or two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 250 mcg per actuation aerosol, 7-g inhaler: NA ($98) | |
| Fluticasone (Flovent Diskus or HFA) | Diskus: 50, 100, or 250 mcg per blister; one or two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | Diskus (60 doses) | |
| 50 mcg per blister aerosol inhaler: NA ($115) | ||||||
| 100 mcg per blister aerosol inhaler: NA ($116) | ||||||
| 250 mcg per blister aerosol inhaler: NA ($155) | ||||||
| HFA: 44, 110, or 220 mcg per spray; two puffs twice per day | HFA | |||||
| 44 mcg per actuation aerosol, 10.6-g inhaler: NA ($120) | ||||||
| 110 mcg per actuation aerosol, 12-g inhaler: NA ($153) | ||||||
| 220 mcg per actuation aerosol, 12-g inhaler: NA ($247) | ||||||
| Mometasone (Asmanex) | 110 or 220 mcg per spray; one or two puffs, divided, once or twice per day | One week | Variable | Second-line prevention of chronic asthma | 60 metered doses | |
| 220 mcg per inhalation aerosol, 0.24-g inhaler: NA ($168) | ||||||
| Leukotriene receptor antagonists | ||||||
| Montelukast (Singulair) | Six to 14 years of age: 5 mg per day or two hours before exercise 15 years and older: 10 mg per day or two hours before exercise | Two hours | 24 hours | Second-line prevention of chronic asthma and allergic rhinitis | 30 5-mg tablets: NA ($157) | |
| 30 10-mg tablets: NA ($152) | ||||||
| Zafirlukast (Accolate) | Five to 11 years of age: 10 mg twice per day, one hour before or two hours after meal 12 years and older: 20 mg twice per day, one hour before or two hours after meal | Unknown | 12 hours | Second-line prevention of chronic asthma | 60 10-mg tablets: NA ($90) | |
| 60 20-mg tablets: NA ($90) | ||||||
| Zileuton, extended-release (Zyflo CR) | Older than 12 years: 1,200 mg twice per day | Two hours | 12 hours | Second-line prevention of chronic asthma | 120 600-mg tablets: NA ($610) | |
| Anticholinergics | ||||||
| Ipratropium (Atrovent HFA) | 17 mcg per spray: two to four puffs, 15 to 30 minutes before exercise and as needed | 15 minutes | Two to four hours | Third-line prevention of acute asthma | 17 mcg per actuation aerosol, 12.9-g inhaler: NA ($172) | |
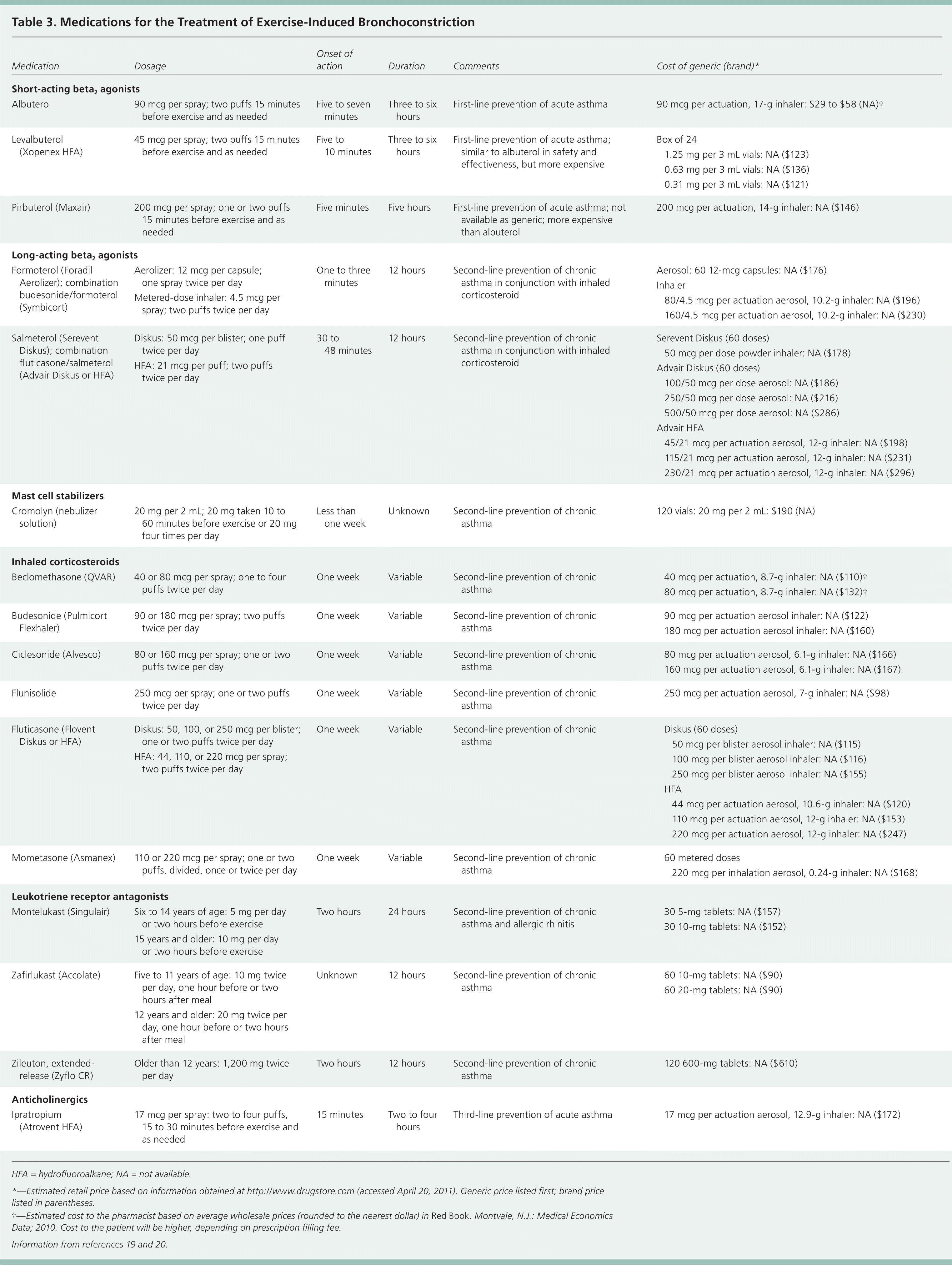
Beta2Agonists. There are two types of inhaled beta2 agonists: short-acting and long-acting. Short-acting beta2 agonists are recommended first-line treatment in the management of EIB, preventively and for acute symptoms.2,14,21 They should be used 15 minutes before exercise, typically have a peak action of 15 to 60 minutes, and last approximately three hours. There is growing concern about the development of tachyphylaxis with daily use of short-acting beta2 agonists; therefore, they should be used only before more strenuous workouts or before competition.5 Although long-acting beta2 agonists have been shown to be effective in persons with EIB,2 the U.S. Food and Drug Administration has recommended that they not be used in persons with asthma unless there is concomitant use of a controller medication, such as inhaled corticosteroids.22 Concurrent use of inhaled corticosteroids and long-acting beta2 agonists has been shown to be effective and superior to use of inhaled corticosteroids alone in managing EIB.23
Mast Cell Stabilizers. Mast cell stabilizers have been shown to be more effective than anticholinergics but less effective than short-acting beta2 agonists for managing EIB.24 Mast cell stabilizers should be used 15 to 20 minutes before exercise. Metered-dose inhalers have been discontinued because they are difficult to manufacture without chlorofluorocarbon propellants; however, cromolyn is still available as a nebulized solution.
Inhaled Corticosteroids. Inhaled corticosteroids are considered controller medications and are the mainstay of treatment in patients with persistent asthma.2,14 A meta-analysis showed that use of inhaled corticosteroids for four weeks or more reduced the percentage decrease in FEV1 after exercise.25 There is a paucity of studies comparing inhaled corticosteroids with other treatments for EIB.
Leukotriene Receptor Antagonists. Leukotriene receptor antagonists have been shown to have a persistent benefit against EIB.14,26 Montelukast (Singulair) has an onset of action within two hours and continued EIB preventive benefit up to 24 hours after a single oral dose.27 Compared with salmeterol (Serevent), montelukast is equally effective at preventing EIB at two hours and at eight and one-half hours, but montelukast is more effective at 24 hours.21 Short-acting beta2 agonists have been shown to be more effective than montelukast in the prevention of EIB.28 Use of montelukast has not been shown to cause tachyphylaxis.29
Other Agents. Ipratropium (Atrovent) is an anticholinergic that provides some protection against EIB but is not as effective as short-acting beta2 agonists or leukotriene receptor antagonists.14 Inhaled heparin30 and furosemide31 (Lasix) have been shown to be effective for treating EIB, but only small sample sizes have been used in studies.
A Practical Approach to the Patient
Chronic, persistent asthma should be treated according to the National Asthma Education and Prevention Program guidelines.2 In athletes with confirmed EIB, a reasonable approach is to start with a short-acting beta2 agonist before exercise (Figure 1). If regular dosing of a short-acting beta2 agonist is needed, or if EIB is not controlled with short-acting beta2 agonists, a second-line agent (e.g., leukotriene receptor antagonist, mast cell stabilizer, inhaled corticosteroid with or without a long-acting beta2 agonist) can be added. Inhaled corticosteroids and leukotriene receptor antagonists are the preferred agents in persons with underlying asthma. Leukotriene receptor antagonists are preferred in persons with allergic rhinitis. When prescribing medications to high-level athletes (e.g., those who participate in the National Collegiate Athletic Association or the Olympics), physicians should be aware of which medicines require a waiver (Table 432,33 ). Patients should be reassessed periodically; if a satisfactory response is not achieved, the diagnosis of EIB should be reconsidered.
| Medication class | NCAA | USOC |
|---|---|---|
| Anticholinergics | Not prohibited | Not prohibited |
| Inhaled beta2 agonists | Permitted only by prescription | Salmeterol (Serevent) and albuterol: athletes must declare use |
| All other inhaled beta2 agonists: therapeutic use exemption is needed | ||
| Inhaled corticosteroids | Not prohibited | Declaration of use required in competition |
| Leukotriene receptor antagonists | Not prohibited | Not prohibited |
| Mast cell stabilizers | Not prohibited | Not prohibited |
NCAA = National Collegiate Athletic Association; USOC = United States Olympic Committee.
Data Sources: A search was performed using the Agency for Healthcare Research and Quality, Cochrane Database of Systematic Reviews, the U.S. Preventive Services Task Force, UpToDate, and the National Guideline Clearinghouse. Search date: May 3, 2010. A PubMed search was performed on May 7 and May 17, 2010. For each source, the following keywords were used: exercise-induced asthma, exercise-induced bronchoconstriction, exercise-induced bronchospasm, exercise-induced, and asthma.
