
Am Fam Physician. 2011;84(4):444-445
Summary of Recommendations and Evidence
The U.S. Preventive Services Task Force (USPSTF) recommends against screening for testicular cancer in adolescent or adult males (Table 1). D recommendation.
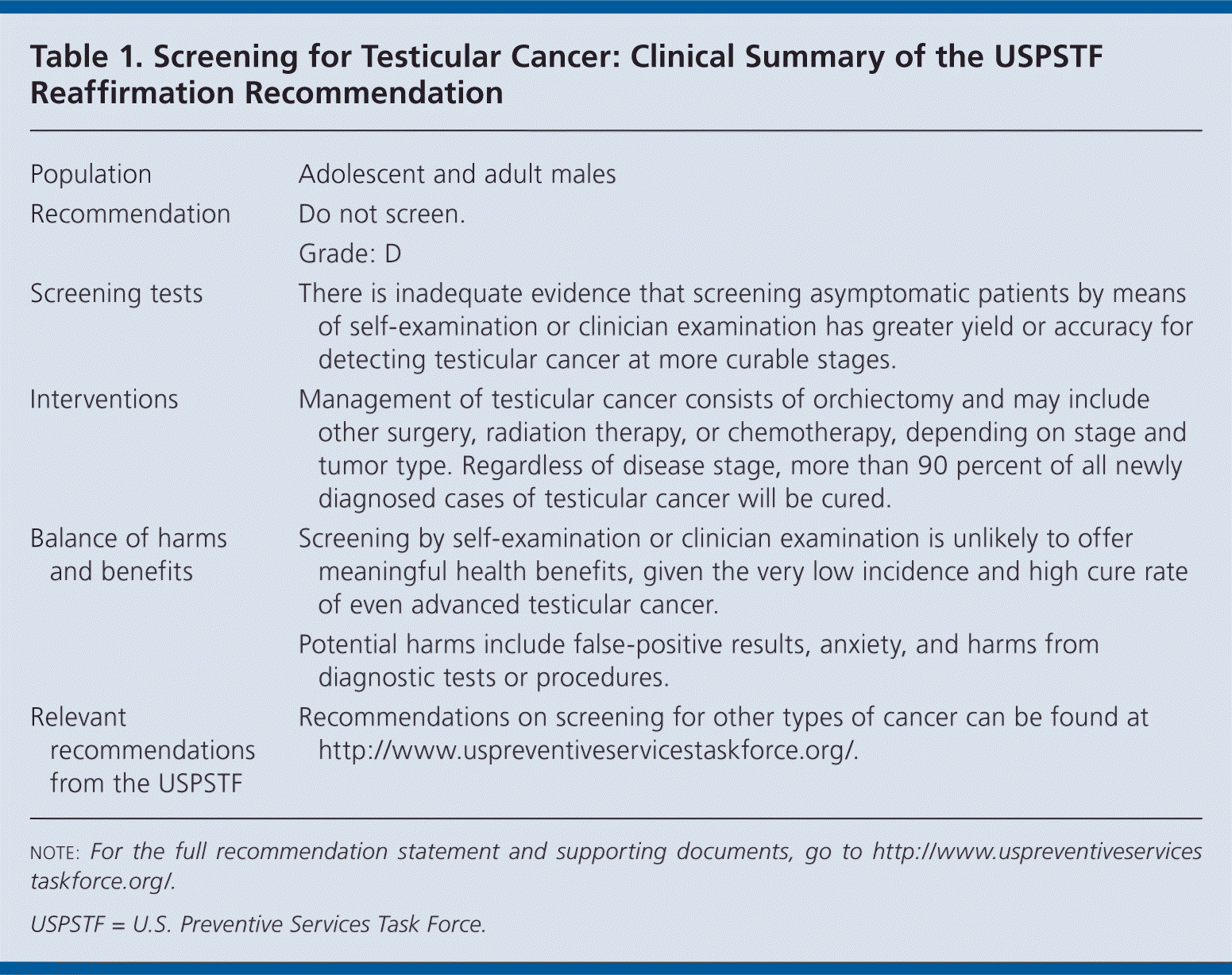
| Population | Adolescent and adult males |
| Recommendation | Do not screen. |
| Grade: D | |
| Screening tests | There is inadequate evidence that screening asymptomatic patients by means of self-examination or clinician examination has greater yield or accuracy for detecting testicular cancer at more curable stages. |
| Interventions | Management of testicular cancer consists of orchiectomy and may include other surgery, radiation therapy, or chemotherapy, depending on stage and tumor type. Regardless of disease stage, more than 90 percent of all newly diagnosed cases of testicular cancer will be cured. |
| Balance of harms and benefits | Screening by self-examination or clinician examination is unlikely to offer meaningful health benefits, given the very low incidence and high cure rate of even advanced testicular cancer. |
| Potential harms include false-positive results, anxiety, and harms from diagnostic tests or procedures. | |
| Relevant recommendations from the USPSTF | Recommendations on screening for other types of cancer can be found at http://www.uspreventiveservicestaskforce.org/. |
Rationale
Importance. Testicular cancer (a primary germ-cell tumor of the testis) is the most common cancer among males 15 to 34 years of age. However, with an annual incidence rate of 5.4 cases per 100,000 males, testicular cancer is relatively rare compared with other types of cancer.
Detection. Most cases of testicular cancer are discovered accidentally by patients or their partners. There is inadequate evidence that screening by clinician examination or patient self-examination has a higher yield or greater accuracy for detecting testicular cancer at earlier (and more curable) stages.
Benefits of detection and early intervention. Based on the low incidence of this condition and favorable outcomes of treatment, even in cases of advanced disease, there is adequate evidence that the benefits of screening for testicular cancer are small to none.
Harms of detection and early intervention. Potential harms associated with screening for testicular cancer include false-positive results, anxiety, and harms from diagnostic tests or procedures. The USPSTF found no new evidence on potential harms of screening and concluded that these harms are no greater than small.
USPSTF assessment. The USPSTF concludes that there is moderate certainty that screening for testicular cancer has no net benefit.
Clinical Considerations
Patient population. This recommendation applies to asymptomatic adolescent or adult males. The USPSTF did not review the evidence for screening males with a history of cryptorchidism.
Screening tests. The sensitivity, specificity, and positive predictive value of testicular examination in asymptomatic patients are unknown. Screening examinations performed by patients or clinicians are unlikely to provide meaningful health benefits because of the low incidence and high survival rate of testicular cancer, even when it is detected at symptomatic stages.1
Treatment. Management of testicular cancer consists of orchiectomy and may include other surgery, radiation therapy, and chemotherapy, depending on the disease stage and tumor type. Regardless of disease stage, more than 90 percent of all newly diagnosed cases of testicular cancer will be cured.2
Useful resources. The National Cancer Institute's Physician Data Query, available at http://www.cancer.gov/cancertopics/pdq, is a comprehensive database that contains summaries on a wide range of cancer-related topics for health professionals and patients, including testicular cancer screening and treatment.
