Am Fam Physician. 2011;84(7):793-800
A more recent article on heart murmurs in children is available.
Author disclosure: No relevant financial affiliations to disclose.
Heart murmurs are common in healthy infants, children, and adolescents. Although most are not pathologic, a murmur may be the sole manifestation of serious heart disease. Historical elements that suggest pathology include family history of sudden cardiac death or congenital heart disease, in utero exposure to certain medications or alcohol, maternal diabetes mellitus, history of rheumatic fever or Kawasaki disease, and certain genetic disorders. Physical examination should focus on vital signs; age-appropriate exercise capacity; respiratory or gastrointestinal manifestations of congestive heart failure; and a thorough cardiovascular examination, including features of the murmur, assessment of peripheral perfusion, and auscultation over the heart valves. Red flags that increase the likelihood of a pathologic murmur include a holosystolic or diastolic murmur, grade 3 or higher murmur, harsh quality, an abnormal S2, maximal murmur intensity at the upper left sternal border, a systolic click, or increased intensity when the patient stands. Electrocardiography and chest radiography rarely assist in the diagnosis. Referral to a pediatric cardiologist is recommended for patients with any other abnormal physical examination findings, a history of conditions that increase the likelihood of structural heart disease, symptoms suggesting underlying cardiac disease, or when a specific innocent murmur cannot be identified by the family physician. Echocardiography provides a definitive diagnosis and is recommended for evaluation of any potentially pathologic murmur, and for evaluation of neonatal heart murmurs because these are more likely to be manifestations of structural heart disease.
Heart murmurs are common in asymptomatic, otherwise healthy children. These murmurs are often innocent and result from the normal patterns of blood flow through the heart and vessels.1 However, a heart murmur may be the sole finding in children with structural heart disease; therefore, a thorough evaluation is necessary.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Structural heart disease is more likely when the murmur is holosystolic, diastolic, grade 3 or higher, or associated with a systolic click; when it increases in intensity with standing; or when it has a harsh quality. | C | 6, 10, 25 |
| Chest radiography and electrocardiography rarely assist in the diagnosis of heart murmurs in children. | B | 5, 6, 29–33 |
| Family physicians should order echocardiography or consider referral to a pediatric cardiologist for newborns with a heart murmur, even if the child is asymptomatic, because of the higher prevalence of structural heart lesions in this population. | B | 28, 43 |
Incidence and Prevalence
History
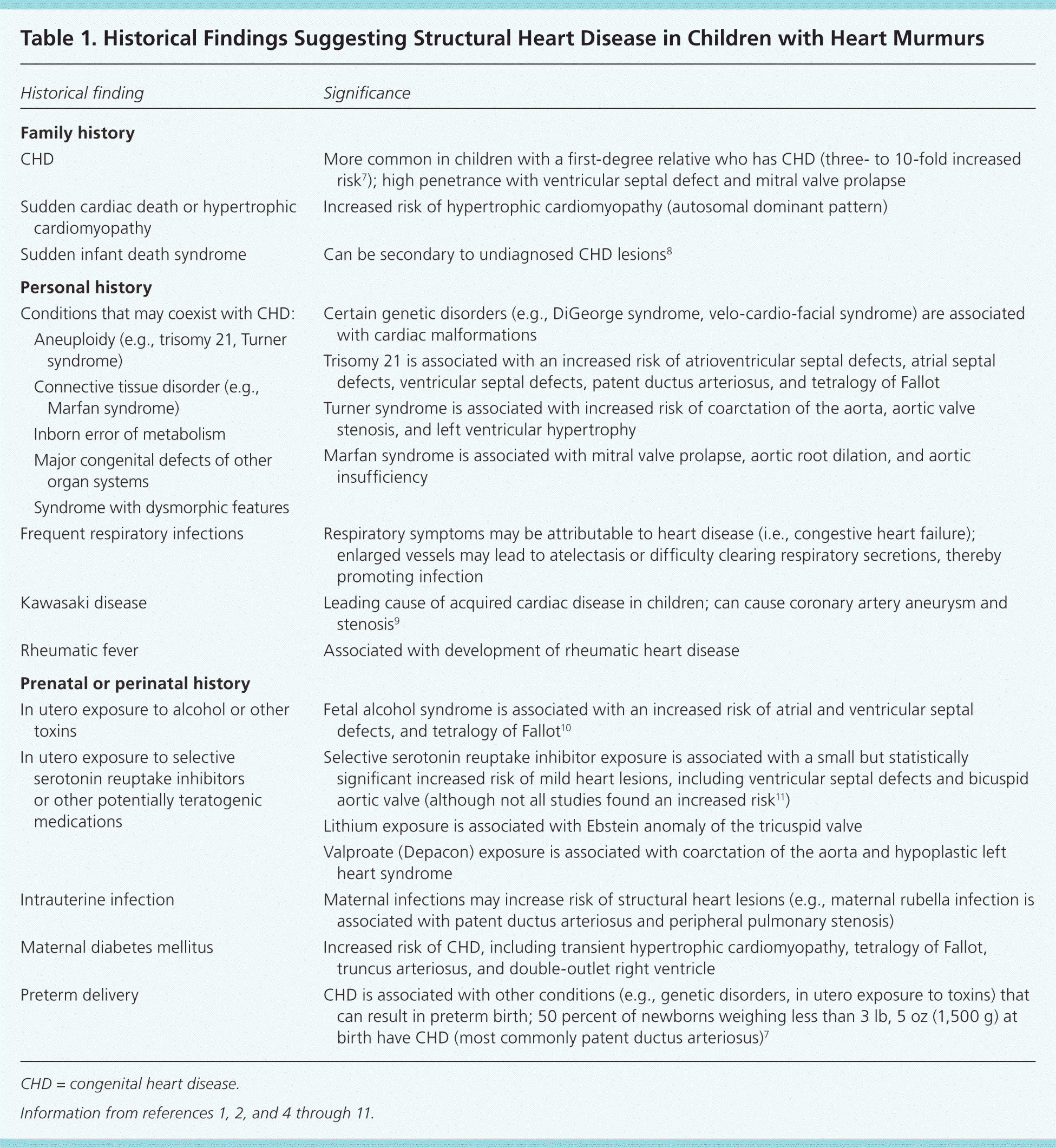
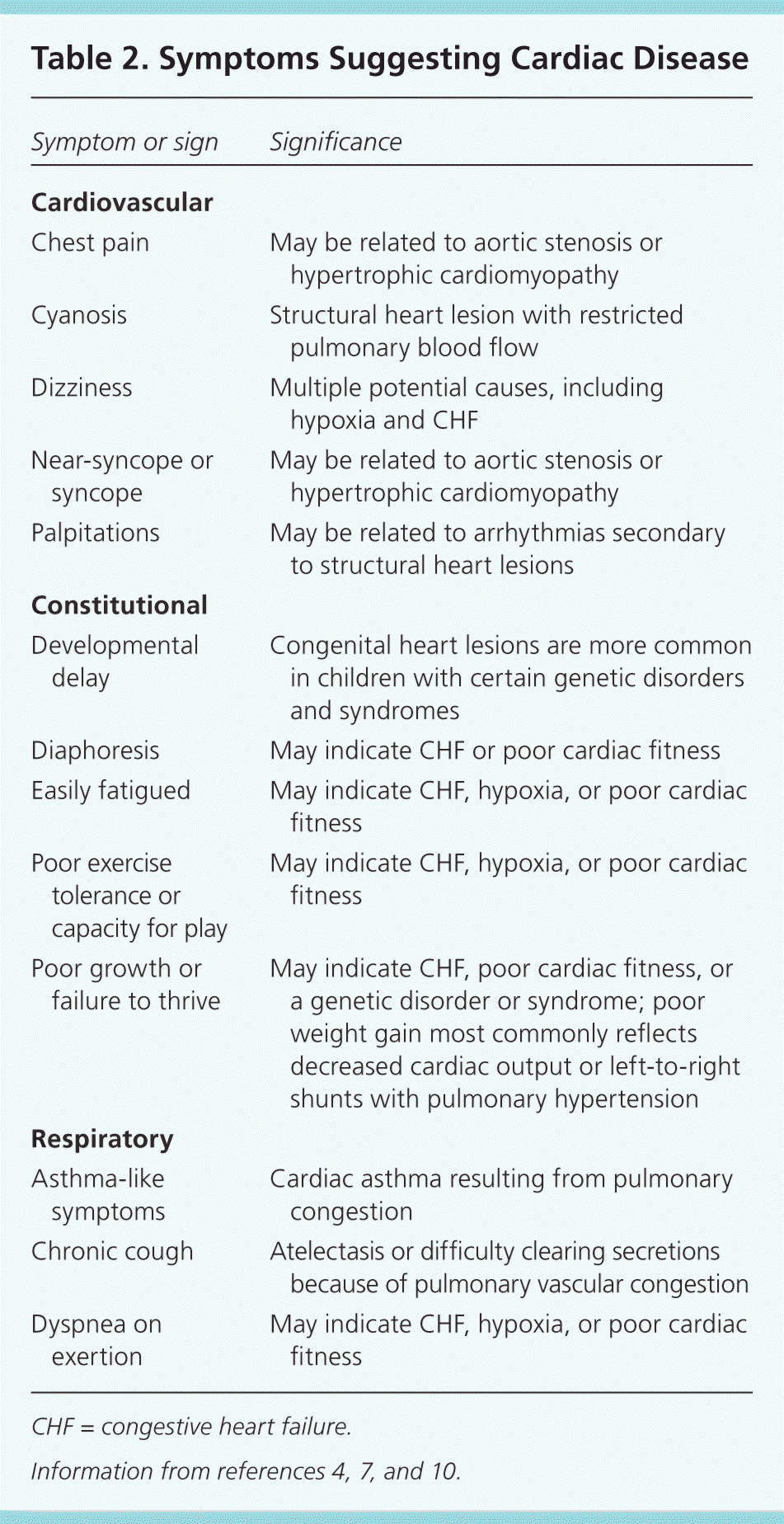
Certain historical features suggest possible structural heart disease (Table 1).1,2,4–11 Cardiovascular signs and symptoms can be non-specific (e.g., poor feeding, failure to thrive) or specific (e.g., chest pain, palpitations), and can help identify children who are likely to have structural heart disease (Table 2).4,7,10
| Historical finding | Significance | |
|---|---|---|
| Family history | ||
| CHD | More common in children with a first-degree relative who has CHD (three- to 10-fold increased risk7); high penetrance with ventricular septal defect and mitral valve prolapse | |
| Sudden cardiac death or hypertrophic cardiomyopathy | Increased risk of hypertrophic cardiomyopathy (autosomal dominant pattern) | |
| Sudden infant death syndrome | Can be secondary to undiagnosed CHD lesions8 | |
| Personal history | ||
| Conditions that may coexist with CHD: | Certain genetic disorders (e.g., DiGeorge syndrome, velo-cardio-facial syndrome) are associated with cardiac malformations | |
| Aneuploidy (e.g., trisomy 21, Turner syndrome) | ||
| Trisomy 21 is associated with an increased risk of atrioventricular septal defects, atrial septal defects, ventricular septal defects, patent ductus arteriosus, and tetralogy of Fallot | ||
| Connective tissue disorder (e.g., Marfan syndrome) | ||
| Turner syndrome is associated with increased risk of coarctation of the aorta, aortic valve stenosis, and left ventricular hypertrophy | ||
| Inborn error of metabolism | ||
| Marfan syndrome is associated with mitral valve prolapse, aortic root dilation, and aortic insufficiency | ||
| Major congenital defects of other organ systems | ||
| Syndrome with dysmorphic features | ||
| Frequent respiratory infections | Respiratory symptoms may be attributable to heart disease (i.e., congestive heart failure); enlarged vessels may lead to atelectasis or difficulty clearing respiratory secretions, thereby promoting infection | |
| Kawasaki disease | Leading cause of acquired cardiac disease in children; can cause coronary artery aneurysm and stenosis9 | |
| Rheumatic fever | Associated with development of rheumatic heart disease | |
| Prenatal or perinatal history | ||
| In utero exposure to alcohol or other toxins | Fetal alcohol syndrome is associated with an increased risk of atrial and ventricular septal defects, and tetralogy of Fallot10 | |
| In utero exposure to selective serotonin reuptake inhibitors or other potentially teratogenic medications | Selective serotonin reuptake inhibitor exposure is associated with a small but statistically significant increased risk of mild heart lesions, including ventricular septal defects and bicuspid aortic valve (although not all studies found an increased risk11) | |
| Lithium exposure is associated with Ebstein anomaly of the tricuspid valve | ||
| Valproate (Depacon) exposure is associated with coarctation of the aorta and hypoplastic left heart syndrome | ||
| Intrauterine infection | Maternal infections may increase risk of structural heart lesions (e.g., maternal rubella infection is associated with patent ductus arteriosus and peripheral pulmonary stenosis) | |
| Maternal diabetes mellitus | Increased risk of CHD, including transient hypertrophic cardiomyopathy, tetralogy of Fallot, truncus arteriosus, and double-outlet right ventricle | |
| Preterm delivery | CHD is associated with other conditions (e.g., genetic disorders, in utero exposure to toxins) that can result in preterm birth; 50 percent of newborns weighing less than 3 lb, 5 oz (1,500 g) at birth have CHD (most commonly patent ductus arteriosus)7 | |
| Symptom or sign | Significance |
|---|---|
| Cardiovascular | |
| Chest pain | May be related to aortic stenosis or hypertrophic cardiomyopathy |
| Cyanosis | Structural heart lesion with restricted pulmonary blood flow |
| Dizziness | Multiple potential causes, including hypoxia and CHF |
| Near-syncope or syncope | May be related to aortic stenosis or hypertrophic cardiomyopathy |
| Palpitations | May be related to arrhythmias secondary to structural heart lesions |
| Constitutional | |
| Developmental delay | Congenital heart lesions are more common in children with certain genetic disorders and syndromes |
| Diaphoresis | May indicate CHF or poor cardiac fitness |
| Easily fatigued | May indicate CHF, hypoxia, or poor cardiac fitness |
| Poor exercise tolerance or capacity for play | May indicate CHF, hypoxia, or poor cardiac fitness |
| Poor growth or failure to thrive | May indicate CHF, poor cardiac fitness, or a genetic disorder or syndrome; poor weight gain most commonly reflects decreased cardiac output or left-to-right shunts with pulmonary hypertension |
| Respiratory | |
| Asthma-like symptoms | Cardiac asthma resulting from pulmonary congestion |
| Chronic cough | Atelectasis or difficulty clearing secretions because of pulmonary vascular congestion |
| Dyspnea on exertion | May indicate CHF, hypoxia, or poor cardiac fitness |
In infants, feeding difficulties may be the first sign of congestive heart failure, which is present in approximately one-third of infants and children with CHD.4 The most common symptoms in a series of children presenting to the emergency department with acute heart failure included dyspnea (74 percent), nausea and vomiting (60 percent), fatigue (56 percent), and cough (40 percent).12
Exercise tolerance should be assessed in an age-appropriate fashion. Parents of infants should be asked about their child's ability to play and the duration and vigor of feeding; parents of older children should compare their child's ability to participate in team sports with that of peers.4 Chest pain is rarely a presenting symptom of cardiac disease in children.13,14 In a pediatric cardiology clinic, chest pain or syncope prompted consultation in approximately 10 percent of children; only 11 percent of those with chest pain and 5 percent of those with syncope had cardiac disease.14 A high degree of suspicion is necessary to detect underlying cardiac disease in children who report exertional syncope or chest pain, or who have a family history of hypertrophic cardiomyopathy.1,13,14
Physical Examination
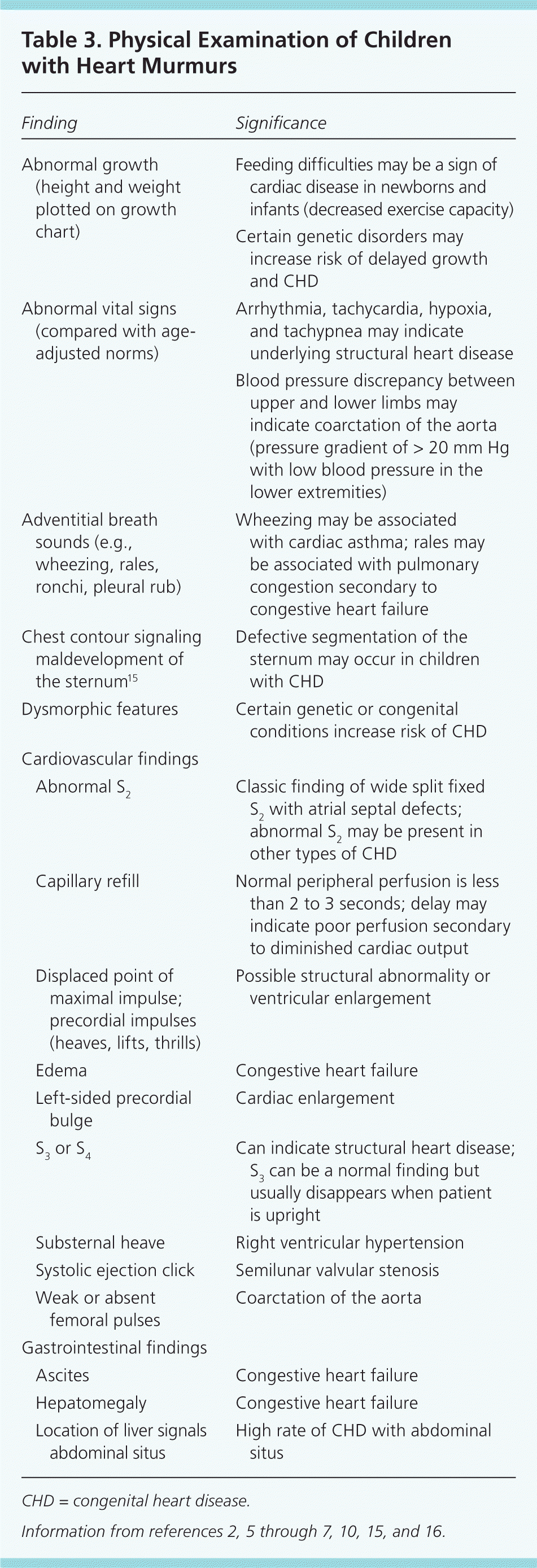
The patient's vital signs should be compared with age-established norms (available at http://www.cc.nih.gov/ccc/pedweb/pedsstaff/age.html), and a focused examination of the respiratory, cardiovascular, and gastrointestinal systems should be performed5 (Table 32 ,5–7,10,15,16 ). Congenital anomalies of other organ systems may be associated with CHD in up to 25 percent of patients.6 The child's appearance, activity level, color, and respiratory effort should be assessed, and the neck should be examined for prominent vessels, abnormal pulsations, and bruits.1 Jugular venous distension is rare in children.4 The chest wall should be inspected for abnormalities of the sternum, which can be associated with CHD,15 and for abnormal cardiac impulses or thrills.1 The lungs should be auscultated for abnormal breath sounds such as crackles, which may indicate pulmonary congestion, or wheezing, which may indicate cardiac asthma. Abdominal examination should focus on the liver location (seeking abdominal situs) and evaluation for liver enlargement or ascites, which may signal congestive heart failure.5
| Finding | Significance | |
|---|---|---|
| Abnormal growth (height and weight plotted on growth chart) | Feeding difficulties may be a sign of cardiac disease in newborns and infants (decreased exercise capacity) | |
| Certain genetic disorders may increase risk of delayed growth and CHD | ||
| Abnormal vital signs (compared with age-adjusted norms) | Arrhythmia, tachycardia, hypoxia, and tachypnea may indicate underlying structural heart disease | |
| Blood pressure discrepancy between upper and lower limbs may indicate coarctation of the aorta (pressure gradient of > 20 mm Hg with low blood pressure in the lower extremities) | ||
| Adventitial breath sounds (e.g., wheezing, rales, ronchi, pleural rub) | Wheezing may be associated with cardiac asthma; rales may be associated with pulmonary congestion secondary to congestive heart failure | |
| Chest contour signaling maldevelopment of the sternum15 | Defective segmentation of the sternum may occur in children with CHD | |
| Dysmorphic features | Certain genetic or congenital conditions increase risk of CHD | |
| Cardiovascular findings | ||
| Abnormal S2 | Classic finding of wide split fixed S2 with atrial septal defects; abnormal S2 may be present in other types of CHD | |
| Capillary refill | Normal peripheral perfusion is less than 2 to 3 seconds; delay may indicate poor perfusion secondary to diminished cardiac output | |
| Displaced point of maximal impulse; precordial impulses (heaves, lifts, thrills) | Possible structural abnormality or ventricular enlargement | |
| Edema | Congestive heart failure | |
| Left-sided precordial bulge | Cardiac enlargement | |
| S3 or S4 | Can indicate structural heart disease; S3 can be a normal finding but usually disappears when patient is upright | |
| Substernal heave | Right ventricular hypertension | |
| Systolic ejection click | Semilunar valvular stenosis | |
| Weak or absent femoral pulses | Coarctation of the aorta | |
| Gastrointestinal findings | ||
| Ascites | Congestive heart failure | |
| Hepatomegaly | Congestive heart failure | |
| Location of liver signals abdominal situs | High rate of CHD with abdominal situs | |
The peripheral pulses should be examined for rate, rhythm, volume, and character, and capillary refill time should be less than three seconds.4 The heart should be auscultated over the tricuspid, pulmonary, mitral, and aortic areas with the bell and diaphragm of the stethoscope while the patient is supine, sitting, and standing17 (Figure 118 ).Innocent murmurs are produced by the normal flow of blood through the heart. Changing the flow by changing the patient's position (for example, decreasing flow to the heart with the Valsalva maneuver) will change the intensity of the murmur. Young children should be prompted to push out their abdomen against the examiner's hand.1 The physician should listen for normal S1 and S2; a wide fixed split S2 is characteristic of an atrial septal defect.19 Gallops can be a normal finding in adolescents.1
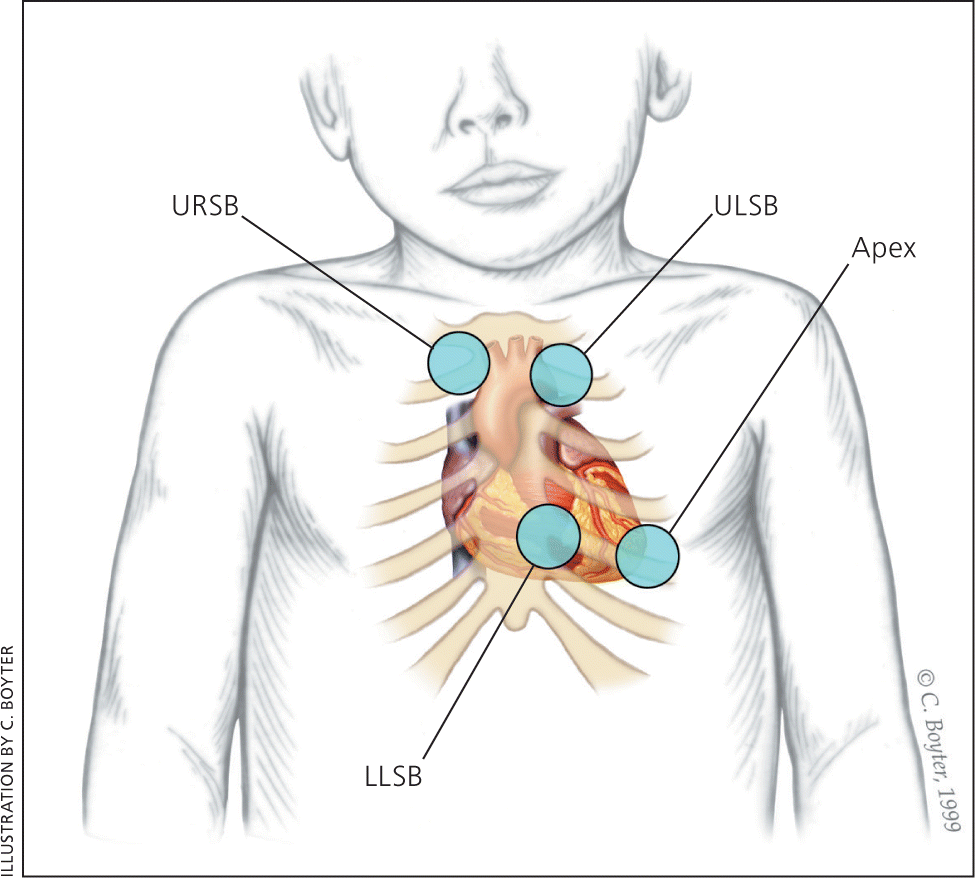
The heart murmur is characterized by its timing during the cardiac cycle; its location, quality, intensity, and pitch (how it sounds); and the presence or absence of clicks1 (Table 45,7,17 and Table 520–23 ). The intensity of heart murmurs is graded from 1 to 6. Grade 1 murmurs are barely audible; grade 2 murmurs are faint but can be heard immediately; grade 3 murmurs can be heard easily and are moderately loud; grade 4 murmurs can be heard easily over a wide area but do not have a palpable thrill; grade 5 murmurs are loud and have a precordial thrill; and grade 6 murmurs are loud enough to hear with the stethoscope raised off the chest.17,24 Certain characteristics of the murmur may be considered red flags, prompting stronger consideration for structural heart disease. These include a holosystolic murmur (odds ratio [OR] of pathologic murmur = 54), grade 3 or higher (OR = 4.8), harsh quality (OR = 2.4), an abnormal S2 (OR = 4.1), maximal intensity at the upper left sternal border (OR = 4.2), a systolic click (OR = 8.3), diastolic murmur, or increased murmur intensity with standing.6,10,25 A decrease or lack of change in the murmur intensity with passive leg elevation (likelihood ratio [LR] = 8.0) or when the child moves from standing to squatting (LR = 4.5) increases the likelihood of hypertrophic cardiomyopathy.26
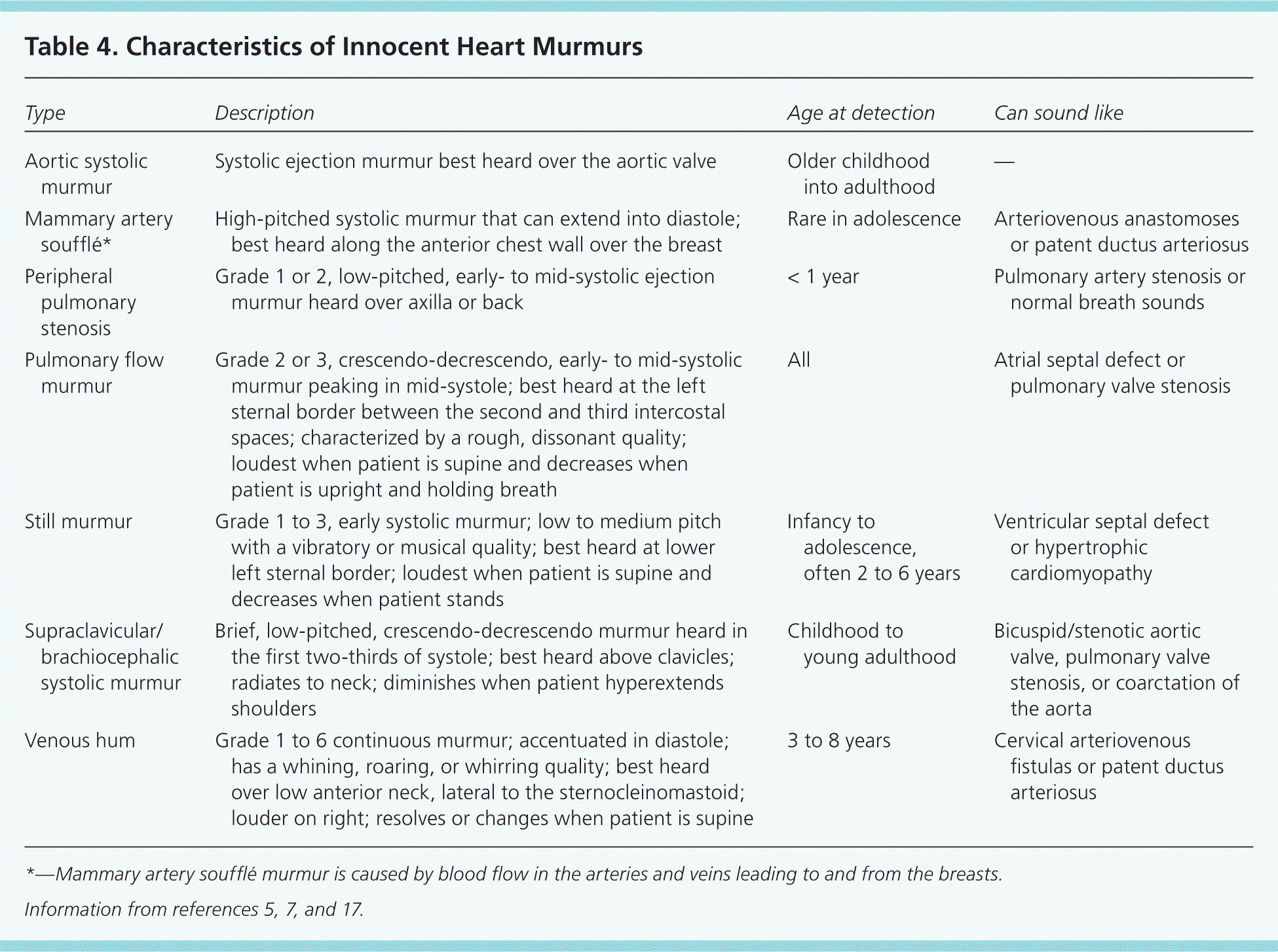
| Type | Description | Age at detection | Can sound like |
|---|---|---|---|
| Aortic systolic murmur | Systolic ejection murmur best heard over the aortic valve | Older childhood into adulthood | — |
| Mammary artery soufflé* | High-pitched systolic murmur that can extend into diastole; best heard along the anterior chest wall over the breast | Rare in adolescence | Arteriovenous anastomoses or patent ductus arteriosus |
| Peripheral pulmonary stenosis | Grade 1 or 2, low-pitched, early- to mid-systolic ejection murmur heard over axilla or back | < 1 year | Pulmonary artery stenosis or normal breath sounds |
| Pulmonary flow murmur | Grade 2 or 3, crescendo-decrescendo, early- to mid-systolic murmur peaking in mid-systole; best heard at the left sternal border between the second and third intercostal spaces; characterized by a rough, dissonant quality; loudest when patient is supine and decreases when patient is upright and holding breath | All | Atrial septal defect or pulmonary valve stenosis |
| Still murmur | Grade 1 to 3, early systolic murmur; low to medium pitch with a vibratory or musical quality; best heard at lower left sternal border; loudest when patient is supine and decreases when patient stands | Infancy to adolescence, often 2 to 6 years | Ventricular septal defect or hypertrophic cardiomyopathy |
| Supraclavicular\brachiocephalic systolic murmur | Brief, low-pitched, crescendo-decrescendo murmur heard in the first two-thirds of systole; best heard above clavicles; radiates to neck; diminishes when patient hyperextends shoulders | Childhood to young adulthood | Bicuspid/stenotic aortic valve, pulmonary valve stenosis, or coarctation of the aorta |
| Venous hum | Grade 1 to 6 continuous murmur; accentuated in diastole; has a whining, roaring, or whirring quality; best heard over low anterior neck, lateral to the sternocleinomastoid; louder on right; resolves or changes when patient is supine | 3 to 8 years | Cervical arteriovenous fistulas or patent ductus arteriosus |
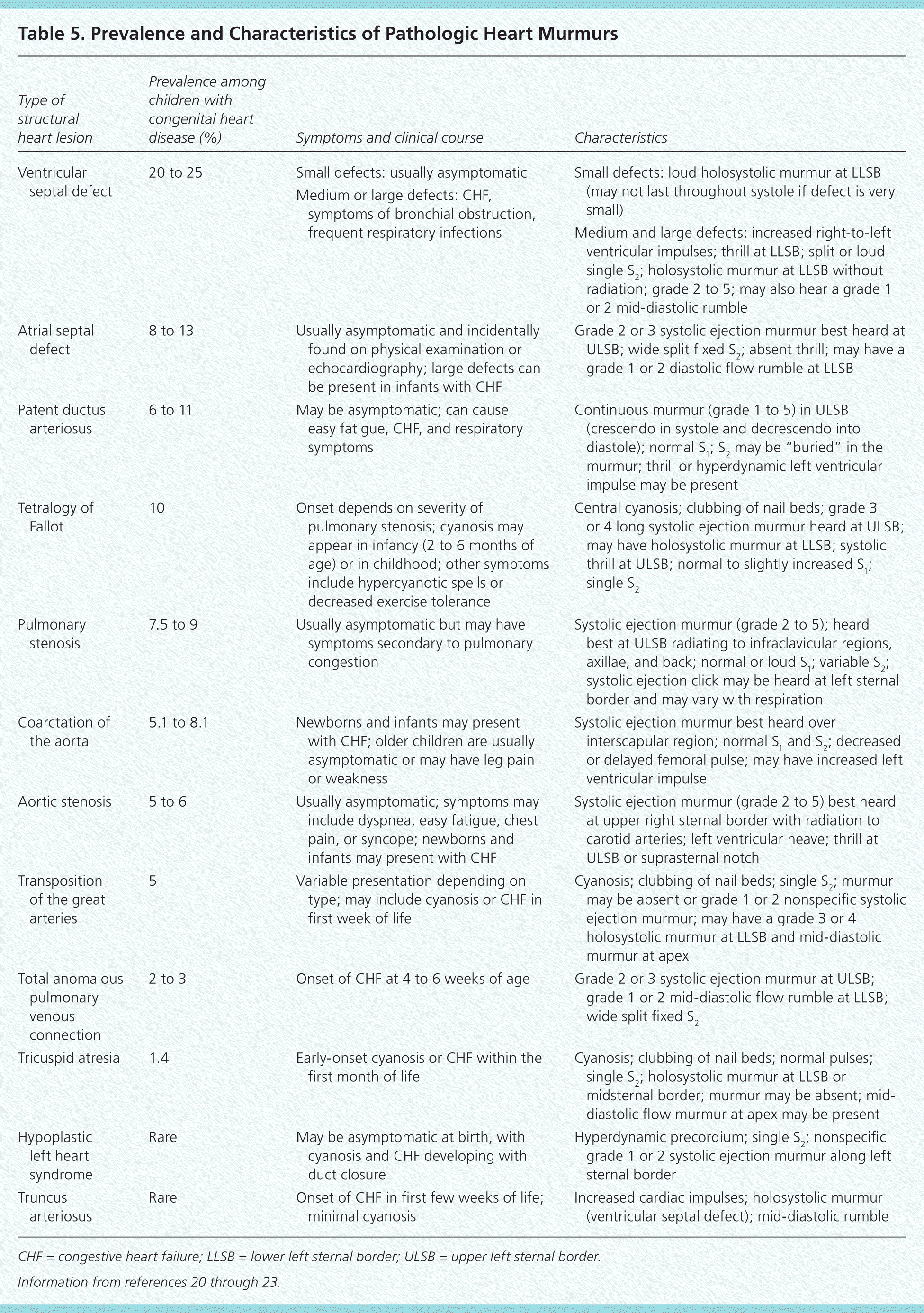
| Type of structural heart lesion | Prevalence among children with congenital heart disease (%) | Symptoms and clinical course | Characteristics |
|---|---|---|---|
| Ventricular septal defect | 20 to 25 | Small defects: usually asymptomatic | Small defects: loud holosystolic murmur at LLSB (may not last throughout systole if defect is very small) |
| Medium or large defects: CHF, symptoms of bronchial obstruction, frequent respiratory infections | |||
| Medium and large defects: increased right-to-left ventricular impulses; thrill at LLSB; split or loud single S2; holosystolic murmur at LLSB without radiation; grade 2 to 5; may also hear a grade 1 or 2 mid-diastolic rumble | |||
| Atrial septal defect | 8 to 13 | Usually asymptomatic and incidentally found on physical examination or echocardiography; large defects can be present in infants with CHF | Grade 2 or 3 systolic ejection murmur best heard at ULSB; wide split fixed S2; absent thrill; may have a grade 1 or 2 diastolic flow rumble at LLSB |
| Patent ductus arteriosus | 6 to 11 | May be asymptomatic; can cause easy fatigue, CHF, and respiratory symptoms | Continuous murmur (grade 1 to 5) in ULSB (crescendo in systole and decrescendo into diastole); normal S1; S2 may be “buried” in the murmur; thrill or hyperdynamic left ventricular impulse may be present |
| Tetralogy of Fallot | 10 | Onset depends on severity of pulmonary stenosis; cyanosis may appear in infancy (2 to 6 months of age) or in childhood; other symptoms include hypercyanotic spells or decreased exercise tolerance | Central cyanosis; clubbing of nail beds; grade 3 or 4 long systolic ejection murmur heard at ULSB; may have holosystolic murmur at LLSB; systolic thrill at ULSB; normal to slightly increased S1; single S2 |
| Pulmonary stenosis | 7.5 to 9 | Usually asymptomatic but may have symptoms secondary to pulmonary congestion | Systolic ejection murmur (grade 2 to 5); heard best at ULSB radiating to infraclavicular regions, axillae, and back; normal or loud S1; variable S2; systolic ejection click may be heard at left sternal border and may vary with respiration |
| Coarctation of the aorta | 5.1 to 8.1 | Newborns and infants may present with CHF; older children are usually asymptomatic or may have leg pain or weakness | Systolic ejection murmur best heard over interscapular region; normal S1 and S2; decreased or delayed femoral pulse; may have increased left ventricular impulse |
| Aortic stenosis | 5 to 6 | Usually asymptomatic; symptoms may include dyspnea, easy fatigue, chest pain, or syncope; newborns and infants may present with CHF | Systolic ejection murmur (grade 2 to 5) best heard at upper right sternal border with radiation to carotid arteries; left ventricular heave; thrill at ULSB or suprasternal notch |
| Transposition of the great arteries | 5 | Variable presentation depending on type; may include cyanosis or CHF in first week of life | Cyanosis; clubbing of nail beds; single S2; murmur may be absent or grade 1 or 2 nonspecific systolic ejection murmur; may have a grade 3 or 4 holosystolic murmur at LLSB and mid-diastolic murmur at apex |
| Total anomalous pulmonary venous connection | 2 to 3 | Onset of CHF at 4 to 6 weeks of age | Grade 2 or 3 systolic ejection murmur at ULSB; grade 1 or 2 mid-diastolic flow rumble at LLSB; wide split fixed S2 |
| Tricuspid atresia | 1.4 | Early-onset cyanosis or CHF within the first month of life | Cyanosis; clubbing of nail beds; normal pulses; single S2; holosystolic murmur at LLSB or midsternal border; murmur may be absent; mid-diastolic flow murmur at apex may be present |
| Hypoplastic left heart syndrome | Rare | May be asymptomatic at birth, with cyanosis and CHF developing with duct closure | Hyperdynamic precordium; single S2; nonspecific grade 1 or 2 systolic ejection murmur along left sternal border |
| Truncus arteriosus | Rare | Onset of CHF in first few weeks of life; minimal cyanosis | Increased cardiac impulses; holosystolic murmur (ventricular septal defect); mid-diastolic rumble |
Characteristics that are more likely to be associated with an innocent murmur include a systolic (rather than diastolic) murmur; soft sound; short duration; musical or low pitch; varying intensity with phases of respiration and posture (louder in supine position); and murmurs that become louder with exercise, anxiety, or fear 17,24 (Table 627 ). The most common innocent murmur is a Still murmur, which is characteristically loudest at the lower left sternal border and has a musical or vibratory quality that is thought to represent vibrations of the left outflow tract.1,5
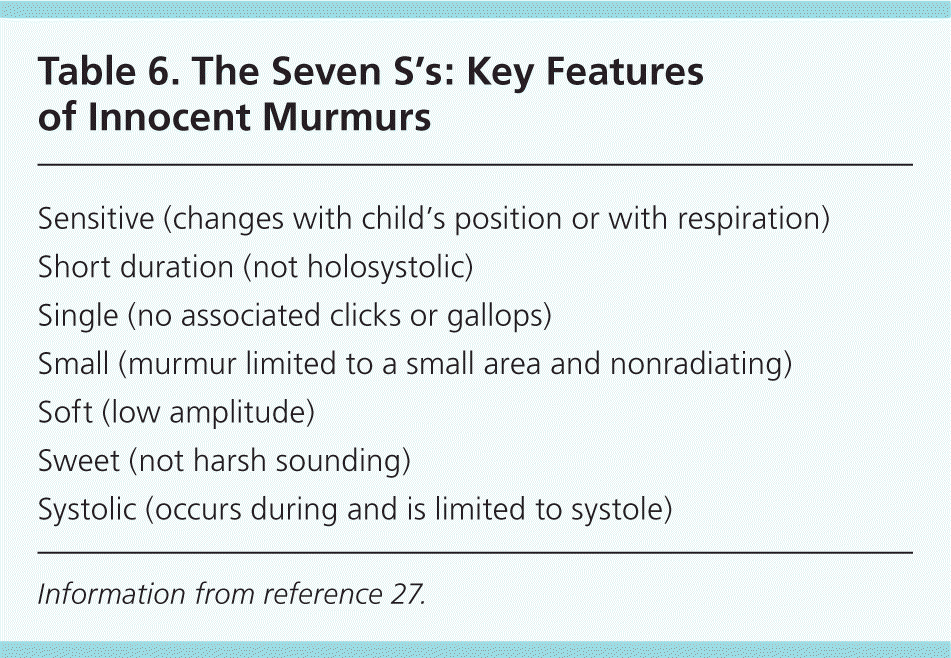
| Sensitive (changes with child's position or with respiration) |
| Short duration (not holosystolic) |
| Single (no associated clicks or gallops) |
| Small (murmur limited to a small area and nonradiating) |
| Soft (low amplitude) |
| Sweet (not harsh sounding) |
| Systolic (occurs during and is limited to systole) |
Auscultation may be less accurate in younger patients, when other signs or symptoms of cardiovascular disease are present, and when findings on radiography or electrocardiography (ECG) are abnormal.28 Online libraries of digital heart sounds are available to familiarize physicians with the characteristics of abnormal heart sounds (Table 7).
| The Auscultation Assistant | |
| Web site: http://www.wilkes.med.ucla.edu/inex.htm | |
| Blaufuss Medical Multimedia Laboratories | |
| Web site: http://www.blaufuss.org | |
| Heart Sounds and Murmurs | |
| Web site: http://www.dundee.ac.uk/medther/Cardiology/hsmur.html | |
| Johns Hopkins University Cardiac Auscultatory Recording Database | |
| Web site: http://www.murmurlab.com/card6/ (registrationrequired) | |
| Texas Heart Institute | |
| Web site: http://www.texasheart.org/education/cme/explore/events/eventdetail_5469.cfm | |
| University of Michigan Heart Sound and Murmur Library | |
| Web site: http://www.med.umich.edu/lrc/psb/heartsounds/index.htm | |
| University of Washington Department of Medicine | |
| Demonstrations: Heart Sounds and Murmurs | |
| Web site: http://depts.washington.edu/physdx/heart/demo.html | |
Role of Diagnostic Testing
Chest radiography and ECG rarely assist in the diagnosis of a heart murmur.5,6,29 Studies in newborns30 and children31 with asymptomatic murmurs have shown that chest radiography does not influence clinical management or assist with diagnosis. A prospective study of 201 newborns who were referred to pediatric cardiologists for evaluation of a heart murmur found that the addition of ECG to clinical assessment did not improve the sensitivity or specificity of detecting structural heart lesions.32 In a study of 128 infants and children who were evaluated for heart murmurs, the addition of ECG and chest radiography to cardiac auscultation was more likely to mislead than assist the physician in making the correct diagnosis.33
In a study of more than 900 children in a pediatric cardiology clinic who had innocent-sounding murmurs, an abnormal finding from the history, physical examination, or diagnostic tests (ECG, chest radiography, or pulse oximetry) was 67 percent sensitive but only 38 percent specific for the presence of a structural heart lesion in infants younger than six weeks, yielding positive and negative LRs very near 1.0 (i.e., no useful diagnostic information).28 In infants older than six weeks, sensitivity increased to 100 percent, but specificity decreased to 28 percent (positive LR = 1.6; negative LR = 0.026). Thus, this information is helpful for ruling out structural causes of an innocent-sounding murmur in infants and children older than six weeks, but it is not helpful in younger infants.
In two separate populations geographically remote from a pediatric cardiologist, phonocardiography (i.e., digital heart sound recordings reviewed by a pediatric cardiologist) had high sensitivity and specificity, and good intraobserver agreement in distinguishing between innocent murmurs and murmurs that were potentially or probably pathologic and that required echocardiography.34,35
Indications for Referral
In children and adolescents, the diagnosis of an innocent heart murmur can be made if four criteria are met: absence of abnormal physical examination findings (except for the murmur); a negative review of systems (i.e., child is asymptomatic); a history that is negative for features that increase the risk of structural heart disease; and characteristic auscultatory features of a specific innocent heart murmur.2,5 These criteria are not appropriate for newborns or infants younger than one year because these patients have a higher rate of asymptomatic structural heart disease.36 When an innocent murmur cannot be definitively diagnosed, the child should be referred for echocardiography, to a pediatric cardiologist, or both.
A study in Oman found that the prevalence of abnormal findings on echocardiography was not significantly different between patients referred by pediatric cardiologists and those referred by primary care physicians.37 However, pediatric cardiologists more accurately detect structural heart lesions in newborns and children with heart murmurs,32,38 and can assist family physicians in the assessment of a suspicious murmur. For both innocent and pathologic murmurs, referral to a pediatric cardiologist for confirmation or clarification of the diagnosis is associated with decreased parental anxiety.39
Neonatal Heart Murmurs
Newborns are at higher risk of having serious structural heart disease that presents as an asymptomatic murmur.6,10 Approximately 1 percent of newborns have a heart murmur, and 31 to 86 percent of these infants have structural heart disease,40–42 including asymptomatic newborns. Because of the higher likelihood of structural heart disease in asymptomatic newborns and young infants with heart murmurs, referral to a pediatric cardiologist and/or for echocardiography is recommended.28,42,43 Even potentially life-threatening heart defects may not be associated with any initial signs or symptoms other than a heart murmur.41,42
The reported sensitivity for detection of a pathologic heart murmur in newborns ranges from 80.5 to 94.9 percent among pediatric cardiologists, with specificity ranging from 25 to 92 percent.32,43 These variations are significant because the lowest specificity corresponds to positive and negative LRs of 1.1 and 0.7, which are uninformative, and the highest specificity corresponds to positive and negative LRs of 10 and 0.21, which are quite accurate. The ability of a pediatric cardiologist to accurately identify pathologic murmurs depends on multiple factors, including his or her confidence in the diagnosis. Echocardiography may not be required in newborns with a heart murmur if a pediatric cardiologist has diagnosed an innocent murmur with a high degree of confidence32; however, it is important to consider the relatively high prevalence of structural heart disease among asymptomatic newborns with a heart murmur.
The evaluation of newborns for CHD may include pulse oximetry after 24 hours of life. Clinical examination of asymptomatic newborns has a sensitivity of 46 percent for detection of CHD; this sensitivity increases to 77 percent when clinical examination is combined with pulse oximetry (with a cutoff of 94 percent).44