A more recent article on adult vaccinations is available.
Am Fam Physician. 2011;84(9):1015-1020
Author disclosure: No relevant financial affiliations to disclose.
Vaccine-preventable diseases contribute significantly to the morbidity and mortality of U.S. adults. The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention updates its recommended adult immunization schedule annually. The most recent updates include the permissive but not routine use of the quadrivalent human papillomavirus vaccine to prevent genital warts in males; a single dose of herpes zoster vaccine for adults 60 years and older, regardless of their history; replacing a single dose of tetanus and diphtheria toxoids (Td) vaccine with tetanus, diphtheria, and acellular pertussis (Tdap) vaccine in adults 19 years and older who have not previously received Tdap; expanding the indications for pneumococcal polyvalent-23 vaccine to include all adults with asthma and all smokers; annual seasonal influenza vaccination for all adults; and booster doses of meningococcal vaccine for adults with high-risk conditions. It is vital for family physicians to implement a systematic approach to adult immunization that is patient-, staff-, and physician-focused.
Vaccine-preventable diseases continue to contribute significantly to the morbidity and mortality of U.S. adults, as the recent multistate outbreak of pertussis that affected more than 9,000 persons demonstrates.1 However, the 2009 National Health Interview Survey 2 showed that rates of vaccination against influenza and pneumococcal disease fell far below the goals of 60 percent set by Healthy People 2010.3 Although rates of pneumococcal coverage in adults 65 years and older remained stable at 60 percent, coverage among high-risk adults 19 to 64 years of age fell to 17.5 percent in 2009, a 7.4 percent absolute decrease from 2008.2 Most of this decrease is the result of expanding the high-risk group to include smokers and persons with asthma. In addition, only slightly more than one-half of adults who had received a tetanus or tetanus/diphtheria booster in the past five years reported receiving the acellular pertussis vaccine.2
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| The quadrivalent human papillomavirus vaccine may be considered in males and females nine to 26 years of age to prevent genital warts and cervical and anal cancers. | A | 6–10 | Reviews and consensus guideline |
| Vaccination against herpes zoster is most effective when given as early as possible after 60 years of age. | C | 15 | Randomized controlled trial |
| Vaccinating adults against pertussis, especially those in high-risk groups (e.g., health care professionals, persons who have close contact with infants younger than 12 months of age), reduces the risk of disease outbreaks. | C | 18–20 | Randomized controlled trials and consensus guideline |
| Annual influenza vaccination is recommended for all persons older than six months. | C | 29 | Specific disease targeting based on observational studies |
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention develops a vaccination schedule for adults that is approved annually by the American Academy of Family Physicians and other professional organizations. Information about these schedules is available at http://cdc.gov/vaccines/. Table 1 provides a summary of the recommendations discussed in this article.4,5
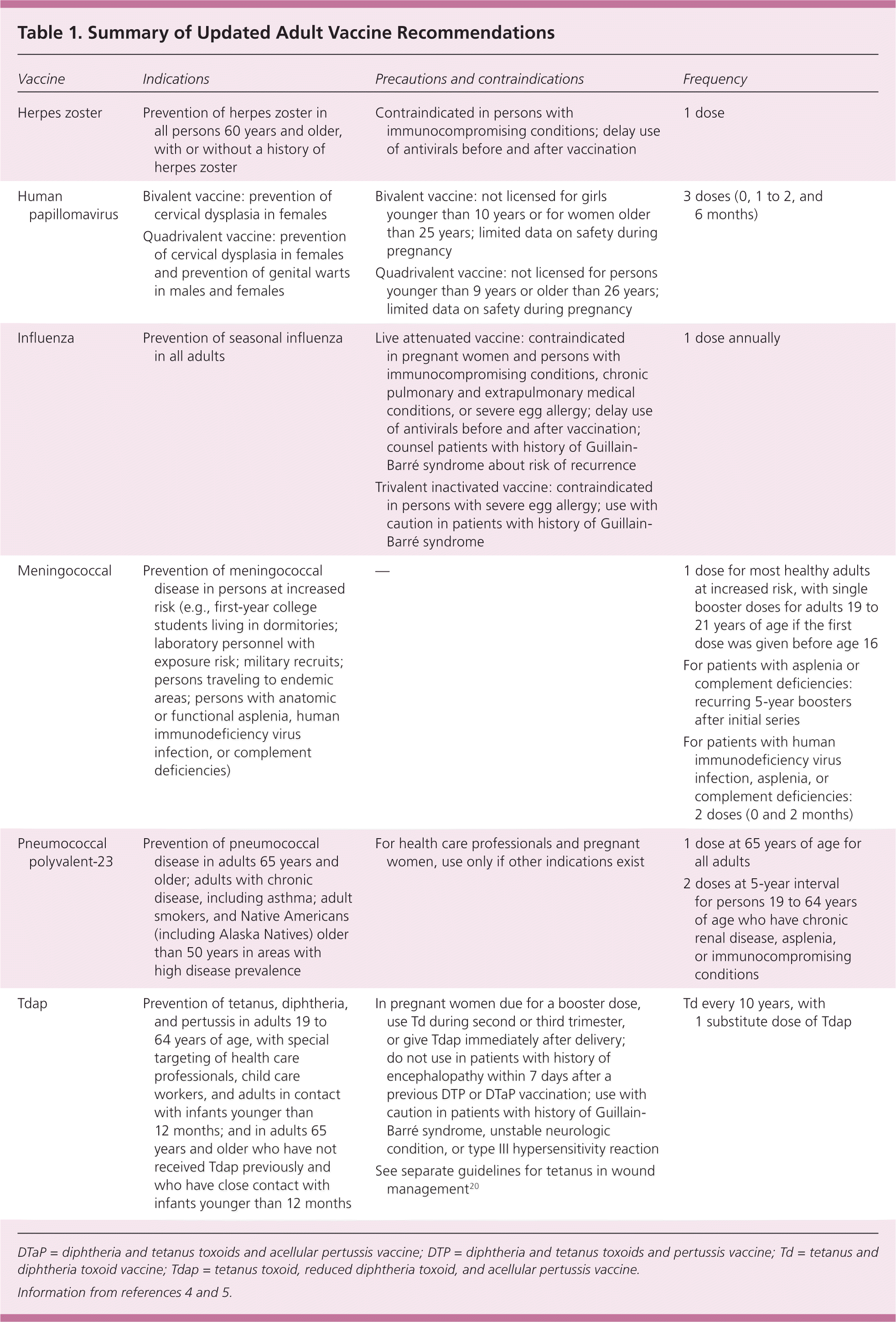
| Vaccine | Indications | Precautions and contraindications | Frequency |
|---|---|---|---|
| Herpes zoster | Prevention of herpes zoster in all persons 60 years and older, with or without a history of herpes zoster | Contraindicated in persons with immunocompromising conditions; delay use of antivirals before and after vaccination | 1 dose |
| Human papillomavirus | Bivalent vaccine: prevention of cervical dysplasia in females | Bivalent vaccine: not licensed for girls younger than 10 years or for women older than 25 years; limited data on safety during pregnancy | 3 doses (0, 1 to 2, and 6 months) |
| Quadrivalent vaccine: prevention of cervical dysplasia in females and prevention of genital warts in males and females | Quadrivalent vaccine: not licensed for persons younger than 9 years or older than 26 years; limited data on safety during pregnancy | ||
| Influenza | Prevention of seasonal influenza in all adults | Live attenuated vaccine: contraindicated in pregnant women and persons with immunocompromising conditions, chronic pulmonary and extrapulmonary medical conditions, or severe egg allergy; delay use of antivirals before and after vaccination; counsel patients with history of Guillain-Barré syndrome about risk of recurrence | 1 dose annually |
| Trivalent inactivated vaccine: contraindicated in persons with severe egg allergy; use with caution in patients with history of Guillain-Barré syndrome | |||
| Meningococcal | Prevention of meningococcal disease in persons at increased risk (e.g., first-year college students living in dormitories; laboratory personnel with exposure risk; military recruits; persons traveling to endemic areas; persons with anatomic or functional asplenia, human immunodeficiency virus infection, or complement deficiencies) | — | 1 dose for most healthy adults at increased risk, with single booster doses for adults 19 to 21 years of age if the first dose was given before age 16 |
| For patients with asplenia or complement deficiencies: recurring 5-year boosters after initial series | |||
| For patients with human immunodeficiency virus infection, asplenia, or complement deficiencies: 2 doses (0 and 2 months) | |||
| Pneumococcal polyvalent-23 | Prevention of pneumococcal disease in adults 65 years and older; adults with chronic disease, including asthma; adult smokers, and Native Americans (including Alaska Natives) older than 50 years in areas with high disease prevalence | For health care professionals and pregnant women, use only if other indications exist | 1 dose at 65 years of age for all adults |
| 2 doses at 5-year interval for persons 19 to 64 years of age who have chronic renal disease, asplenia, or immunocompromising conditions | |||
| Tdap | Prevention of tetanus, diphtheria, and pertussis in adults 19 to 64 years of age, with special targeting of health care professionals, child care workers, and adults in contact with infants younger than 12 months; and in adults 65 years and older who have not received Tdap previously and who have close contact with infants younger than 12 months | In pregnant women due for a booster dose, use Td during second or third trimester, or give Tdap immediately after delivery; do not use in patients with history of encephalopathy within 7 days after a previous DTP or DTaP vaccination; use with caution in patients with history of Guillain-Barré syndrome, unstable neurologic condition, or type III hypersensitivity reaction See separate guidelines for tetanus in wound management20 | Td every 10 years, with 1 substitute dose of Tdap |
New Vaccine Recommendations
HUMAN PAPILLOMAVIRUS
A quadrivalent vaccine against human papillomavirus (HPV) types 6, 11, 16, and 18 was licensed in 2006 for adolescent and young adult women up to 26 years of age; a bivalent vaccine against HPV types 16 and 18 was approved in 2009 for women up to 25 years of age. HPV types 16 and 18 cause 70 percent of cervical cancers, and types 6 and 11 cause 90 percent of genital warts.6 Based on the vaccine's effectiveness in preventing genital warts, in 2010 ACIP recommended the permissive—but not routine—use of the quadrivalent HPV vaccine in males nine to 26 years of age, one year after the U.S. Food and Drug Administration approved it for this use.7 Research suggests that HPV vaccination reduces the risk of anal and oropharyngeal cancers in heterosexual couples and in men who have sex with men.8–10 In December 2010, the license was expanded to include anal cancers and anal intraepithelial neoplasia,11 and ACIP is considering revising its current permissive-use recommendation based on these new data.12 The vaccine is most effective when it is administered before the initiation of sexual activity; efficacy and cost-effectiveness for vaccination after the initiation of sexual activity are being investigated.
HERPES ZOSTER
A large proportion of health care professionals have serologic evidence of previous infection with varicella zoster virus,13 and one-third will go on to develop herpes zoster. The current herpes zoster vaccine (Zostavax) was licensed in 2006; a single dose is recommended for adults 60 years and older, regardless of their history of herpes zoster.14 Vaccine effectiveness decreases with age; therefore, patients should be immunized as soon as possible after 60 years of age.15 The vaccine contains a live virus and is therefore contraindicated in persons with immunocompromising conditions. It must be stored in a freezer.
TETANUS, DIPHTHERIA, AND PERTUSSIS
Pertussis remains endemic in the United States, with cyclic outbreaks every three to five years. Major outbreaks occurred in 2010, with more than 9,200 reported cases in California, Michigan, and Ohio.1,16 The 7,297 cases in California represent the highest incidence of the illness in that state since 1958.17
The first tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) was licensed for adults in 2005.18,19 Current recommendations indicate that Tdap should replace a single dose of tetanus and diphtheria toxoid (Td) booster in adults 19 to 64 years of age who have not received Tdap previously. There is no minimum interval between administration of Td and Tdap. In its 2011 adult immunization recommendations, ACIP recommended that a single dose of Tdap may be given in place of Td for adults 65 years and older who have not received Tdap previously and who have close contact with infants younger than 12 months.5 Administration of Tdap in adults 65 years and older is an off-label use. A single dose of Tdap is also recommended for postpartum women, close contacts of infants younger than 12 months, and previously unvaccinated health care professionals with direct patient contact.20
Vaccines with Expanded Indications
PNEUMOCOCCAL DISEASE
Although there are only limited data from randomized trials showing chronic disease-specific effectiveness,21,22 decreased disease incidence, or decreased all-cause mortality rates23 with the use of the pneumococcal polyvalent-23 vaccine (Pneumovax 23), a 2009 meta-analysis demonstrates its effectiveness against invasive disease.24 The benefits of herd immunity and the high incidence of pneumococcal pneumonia as a cause of death in older adults support the general use of the current polysaccharide vaccine. The 2011 recommendations also include indications for Alaska Natives and Native Americans 50 to 64 years of age who live in areas with an increased risk of invasive pneumococcal disease, and have been expanded to include all persons with asthma and all smokers.25 Studies demonstrate that compared with healthy controls, persons with asthma and smokers have two and four times the risk of invasive pneumococcal disease, respectively.26,27
Other clinical indications remain unchanged: persons with chronic lung disease, chronic cardiovascular disease, diabetes mellitus, chronic liver disease, cirrhosis, alcoholism, functional or anatomic asplenia, immunocompromising conditions, cochlear implants, or cerebrospinal fluid leaks, and residents of nursing homes or long-term care facilities should be vaccinated.25
INFLUENZA
Influenza vaccine has been available since the 1940s in an injectable, inactivated form. An intranasal live attenuated vaccine was licensed in 2003. The high degree of antigenic shift and drift in circulating influenza strains leads to the periodic introduction of strains with high susceptibility in the population, resulting in pandemic spread of disease as in 2009. The year-to-year success of the influenza vaccine depends on the accuracy of the predictive models used to determine the strains expected during an upcoming influenza season. The 2011–2012 trivalent inactivated vaccines include the A/California/7/2009 (H1N1)-like strain that was used in the 2009 pandemic response, since this strain is still circulating.28
In 2010, ACIP expanded its recommendations on influenza vaccination to the widest targets yet, recommending universal vaccination of persons older than six months.29 High-risk groups (i.e., those with chronic illness or immunocompromising conditions, pregnant women, health care professionals, and those in contact with high-risk persons) and older adults should receive priority vaccination any time vaccine supply is limited.29 A high-dose formulation of trivalent inactivated influenza vaccine (Fluzone High-Dose) was licensed in 2009 for persons 65 years and older.30 This vaccine has shown higher immune titers in older adults,31 but it is not preferred over other formulations. Further studies are needed to determine if higher titers lead to a reduction in influenza morbidity. An intradermal formulation of trivalent inactivated influenza vaccine (Fluzone Intradermal) was licensed in 2011 and is an alternative in persons 18 to 64 years of age.
Although several randomized studies found only limited evidence to support the effectiveness of influenza vaccination in persons with chronic diseases (e.g., cystic fibrosis, chronic obstructive pulmonary disease, asthma),32–34 the widespread nature of the infection and the ease of transmission support the promotion of influenza vaccination to all adults.
Vaccines for Specific Risk Groups
HEPATITIS A
Vaccination against hepatitis A should be considered for unvaccinated adults with risk factors for exposure, including those who anticipate having close contact with a child adopted from a country with high or intermediate endemicity during the first 60 days after his or her arrival in the United States.4,5
MENINGOCOCCAL DISEASE
Adults 55 years and younger who require meningococcal vaccination should receive the quadrivalent meningococcal conjugate vaccine; those 56 years and older should receive the quadrivalent meningococcal polysaccharide vaccine. Persons without a functioning spleen and those with persistent complement deficiencies should routinely receive a two-dose series with two months between doses, then booster doses every five years. Persons with human immunodeficiency virus infection do not require booster doses after the initial two-dose series. Other adults who are at increased risk of infection (e.g., first-year college students living in dormitories, laboratory personnel at risk of exposure, travelers to endemic areas) should receive a single dose of vaccine; revaccination is not required unless the first dose was given before 16 years of age.4,5
Safety, Effectiveness, and Best Practices
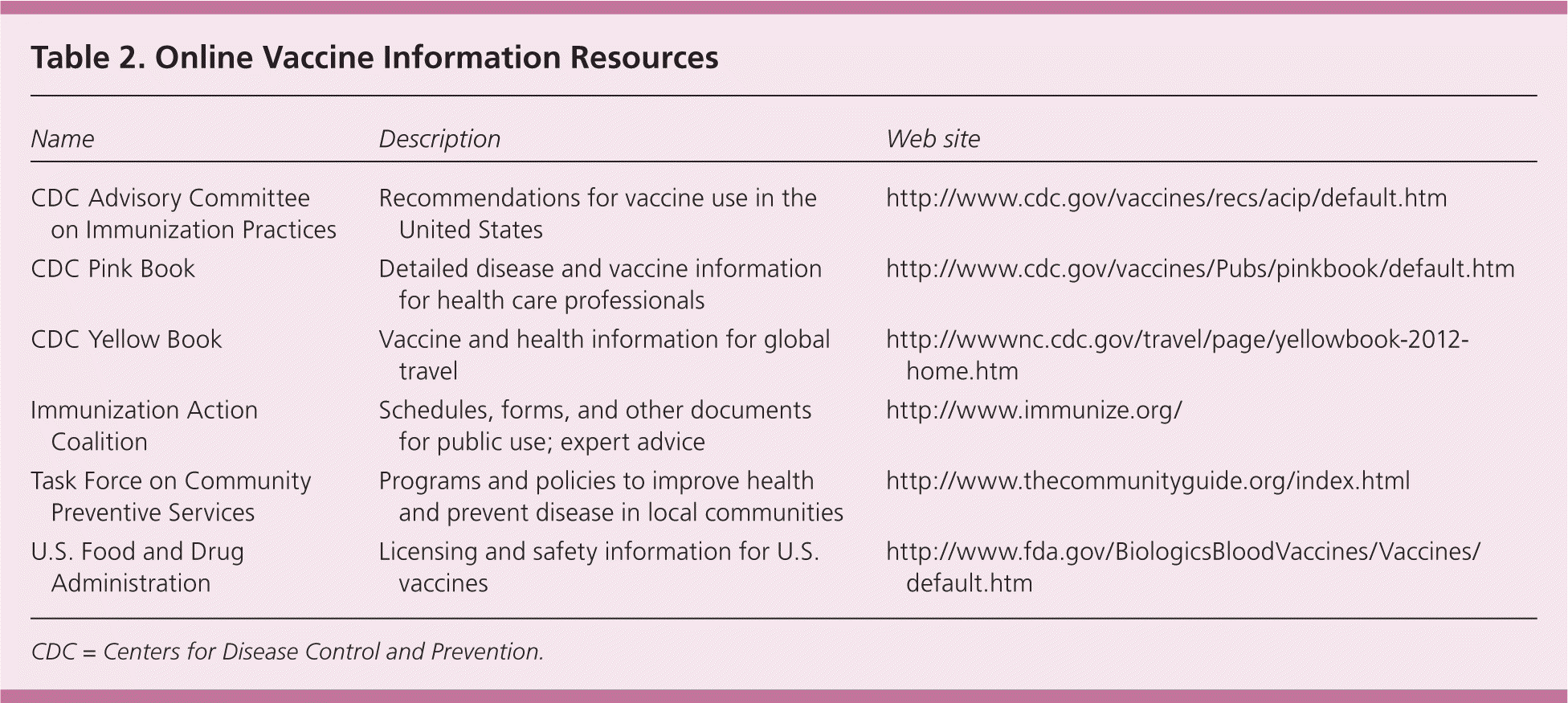
| Name | Description | Web site |
|---|---|---|
| CDC Advisory Committee on Immunization Practices | Recommendations for vaccine use in the United States | http://www.cdc.gov/vaccines/recs/acip/default.htm |
| CDC Pink Book | Detailed disease and vaccine information for health care professionals | http://www.cdc.gov/vaccines/Pubs/pinkbook/default.htm |
| CDC Yellow Book | Vaccine and health information for global travel | http://wwwnc.cdc.gov/travel/page/yellowbook-2012-home.htm |
| Immunization Action Coalition | Schedules, forms, and other documents for public use; expert advice | http://www.immunize.org/ |
| Task Force on Community | Programs and policies to improve health | http://www.thecommunityguide.org/index.html |
| Preventive Services | and prevent disease in local communities | |
| U.S. Food and Drug Administration | Licensing and safety information for U.S. vaccines | http://www.fda.gov/BiologicsBloodVaccines/Vaccines/default.htm |
Various incentive systems have been used to encourage vaccination, and efforts have been made to improve community access to vaccines.37,38 Recall and reminder systems have resulted in increases of up to 20 percent in rates of vaccination against hepatitis B, tetanus, influenza, and pneumococcal disease39; however, significant financial barriers remain. The Institute of Medicine reported in 2003 that only 50 percent of adults 18 to 64 years of age have vaccination coverage through private insurance or public programs.40 Although these coverage rates may improve under current proposals for health care reform, these data highlight challenges that family physicians face in implementing the most current immunization recommendations.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms immunization, adult immunization, vaccine, and vaccine coverage. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the minutes of the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, the Cochrane database, EBSCOhost, the National Health Interview Survey, National Guideline Clearinghouse, the U.S. Preventive Services Task Force, and UpToDate. Search date: December 16, 2010 (updated May 1, 2011).